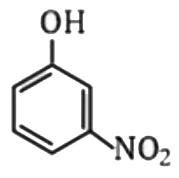
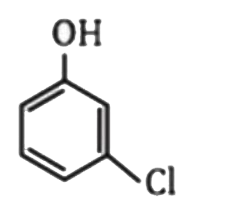
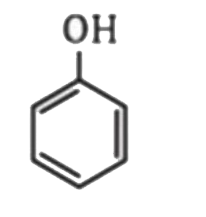
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 36-CHEMISTRY
- For [FeF(6)]^(3-) and [CoF(6)]^(3-), the correct statement is
Text Solution
|
- Consider the following structures (I), (II), (III) and (IV). Whic...
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- Which one of the following is most acidic ?
Text Solution
|
- Which of the following volatile?
Text Solution
|
- Which of the following alkanes will give a single product with a methy...
Text Solution
|
- Which of the following can possibly be used as analgesic without causi...
Text Solution
|
- Consider the following sequence of reactions underset((C(4)H(8)O(3))...
Text Solution
|
- 1, 1- Dichloropropane on hydrolysis gives :
Text Solution
|
- The maximum probability of finding electron in the d(xy) orbital is -
Text Solution
|
- The emf of the cell, Zn|Zn^(2+) (0.01 M)||Fe^(2+) (0.001 M)|Fe at 29...
Text Solution
|
- In the mixture of (NaHCO(3)+Na(2)CO(3)) volume of HCl required is x mL...
Text Solution
|
- A complex is represented as CoCl(3) . XNH(3). Its 0.1 molal solution i...
Text Solution
|
- The shortest distance between two Na^(+) ions in rock - salt arrangeme...
Text Solution
|
- The number of stereoisomers obtained by bromination of trans-2-butene ...
Text Solution
|
- Two moles of an ideal monoatomic gas at 5 bar and 300 K are expanded i...
Text Solution
|
- The pH at the equivalent point for the titration of 0.10 M KH(2)BO(3) ...
Text Solution
|
- Initially 3 moles of 'A' was taken in a 1 L container. The approx. mol...
Text Solution
|
- 0.15g of a subatance dissolved in 15g of solvent boiled at a temperatu...
Text Solution
|
- What hydrogen-like ion has the wavelength difference between the first...
Text Solution
|