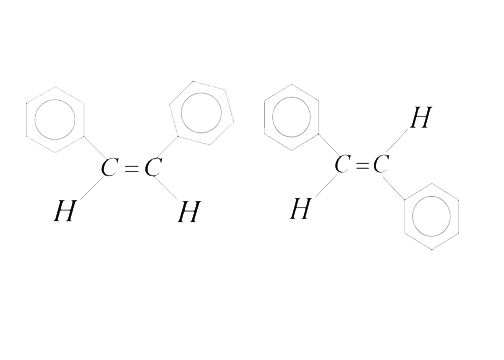
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 41-CHEMISTRY
- Among the following complexes : K(3)[Fe(CN)(6)],[Co(NH(3))(6)]Cl(3) , ...
Text Solution
|
- Flocculation value for As2S3 sol by HCl is 30 m mole L^( –1) . Calcual...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Among the following compounds the most acidic is
Text Solution
|
- The crystal system of a compound with unit cell dimensions a = 0.388 ,...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- For the first order reaction given below select the set having correct...
Text Solution
|
- Amongst the compounds gives, the one that would form a brilliant color...
Text Solution
|
- Passing H(2)S gas into a mixture of Mn^(2+), Ni^(2+), Cu^(2+) and Hg^(...
Text Solution
|
- In psi(321), the sum of angular momentum, spherical nodes and angular ...
Text Solution
|
- The major product obtained in the following reaction is
Text Solution
|
- Select the correct statements. (1) Ferric bromide is obtained when c...
Text Solution
|
- The solubility order for alkali metal fluoride in water is :
Text Solution
|
- Which of the following statements about asprin is not true?
Text Solution
|
- The pH of 0.1 (M) solution of the following salts increases in the ord...
Text Solution
|
- Spin only magnetic moment of the compound Hg[Co(SCN)(4)] is sqrt(x) B....
Text Solution
|
- XeO(4) molecule is tetrahedral having 'x' number of p pi - d pi bonds....
Text Solution
|
- The density of O(2) is 16 at STP. At what temperature (in .^@C) its de...
Text Solution
|
- How many statements are true for the following pair of compounds ? ...
Text Solution
|
- The number of peptide bond(s) in the following molecule is/are
Text Solution
|