A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 54-CHEMISTRY
- Under ambient condition , the total number of gases released products ...
Text Solution
|
- Which of the following is more basic than aniline? .
Text Solution
|
- In a compound C, H, N atoms are present in 9:1:3.5 by weight. Molecula...
Text Solution
|
- For which of the following molecule significant mu ne 0?
Text Solution
|
- The correct statement is
Text Solution
|
- The intermediate product (X) formed in the following reaction is B(2...
Text Solution
|
- The major product of the following reactions is
Text Solution
|
- Product (X and Y) of the following reaction (1 and 2) are (1) unders...
Text Solution
|
- In a galvanic cell, after running the cell for sometimes, the concentr...
Text Solution
|
- Addition of excess aqueous ammonia to a pink coloured aqueous solution...
Text Solution
|
- Given that E(O(2)//H(2)O)^(Theta)= +1.23V, E(S(2)O(8)^(2-)//SO(4)^(2-...
Text Solution
|
- Which of the following is NOT a Correct method of the preparation of b...
Text Solution
|
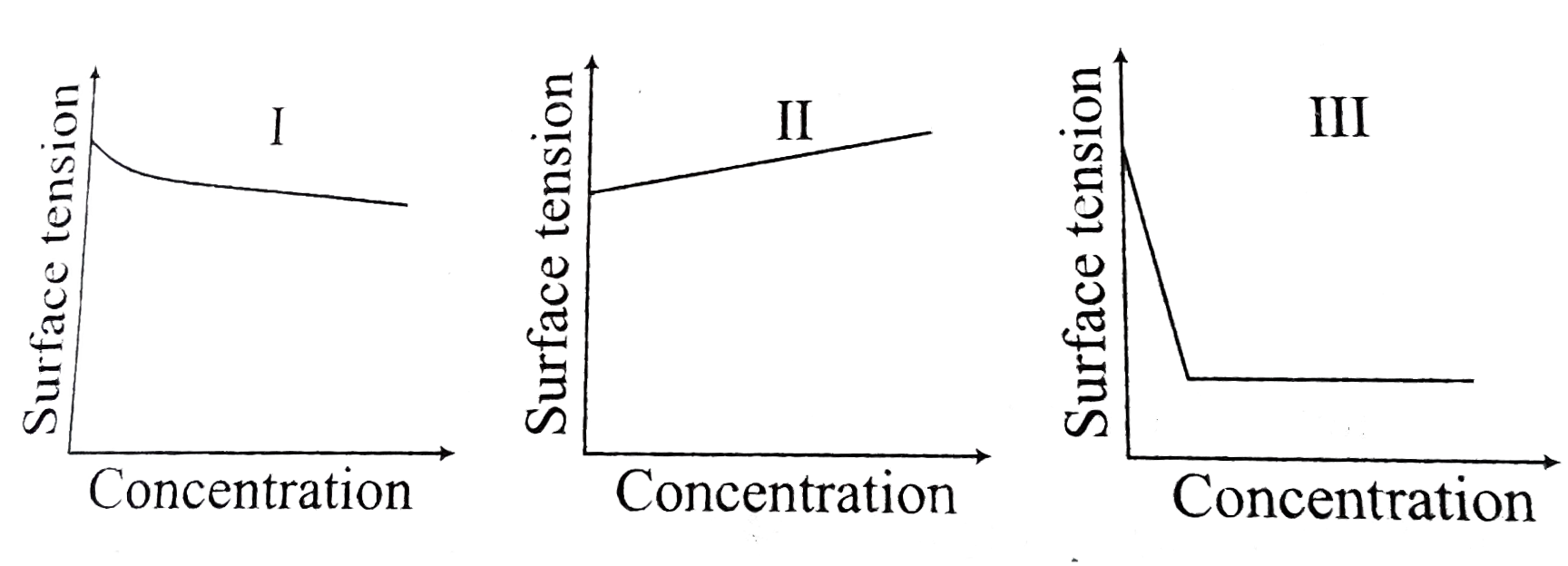
- The equalitative sketches I, II and III given below show the variation...
Text Solution
|
- What is the IUPAC nomenclature of isoprene monomer present in natural ...
Text Solution
|
- What is the relationship between the given structures (look at the arr...
Text Solution
|
- In dilute aqueous H(2)SO(4) the complete diaquadioxalatoferrate (II) i...
Text Solution
|
- A list of species having the formula of XZ(4) is given below XeF(4), S...
Text Solution
|
- Experimentally it was found that a metal oxide in formula M(0.98)O. Me...
Text Solution
|
- In a constant volume calorimeter, 3.5 g of a gas with molecular weight...
Text Solution
|
- In the scheme given below. The total number of intramolecular aldol co...
Text Solution
|