A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 65-CHEMISTRY
- Match the column I with column II and mark the appropriate choice.
Text Solution
|
- The molecular formula of a commercial resin used for exchanging ions i...
Text Solution
|
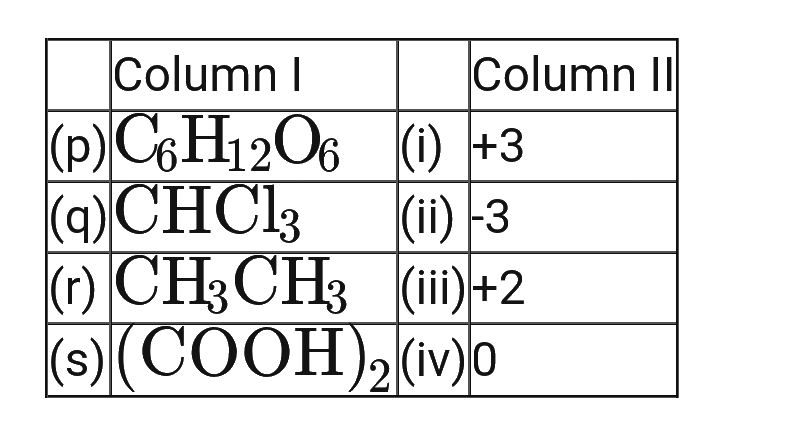
- Match the compounds given in column I with oxidation states of carbon ...
Text Solution
|
- Two plots are shown below between concentration and time t. Which of t...
Text Solution
|
- Consider the following molecules underset("I")(O(2)),underset("II")(O(...
Text Solution
|
- A2 litre vessel is filled with air at 50^(@)C and pressure of 3 atm. T...
Text Solution
|
- The standard enthalpy of formation of gaseous H(2)O at 298 K is -241.8...
Text Solution
|
- The ionisation constant of benzoic acid (PhCOOH) is 6.46 xx 10^(-5) an...
Text Solution
|
- Consider the following reaction. underset(CHO) underset(|) (CHO) + O...
Text Solution
|
- Compound (X) on reduction with LiAlH(4) gives a hydride (Y) containing...
Text Solution
|
- Which of the following will exhibit aromatic character?
Text Solution
|
- Sea water is 3.5%by mass of a salt and has a density 1.04gcm^(-3) at 2...
Text Solution
|
- 0.02 mole of [Co(NH(3))(5)Br]Cl(2) and 0.02" mole of "[Co(NH(3))(5)Cl]...
Text Solution
|
- When an optically active amine 'A' having molecular formula C(4)H(11)N...
Text Solution
|
- Among the following statements about the molecules X and Y, which is i...
Text Solution
|
- For the preparation of a detergent 'P' from benzene, The following ste...
Text Solution
|
- Identify the products (A) and (B) in the reactions. RX+AgCNtoul((A))...
Text Solution
|
- Transition metals make the most efficient catalysts because of their a...
Text Solution
|
- The density of copper is "8.94 g mL"^(-1). Find the charge needed to p...
Text Solution
|
- How many of these acids are monobasic here? H(3)PO(2), H(3)PO(3), H...
Text Solution
|