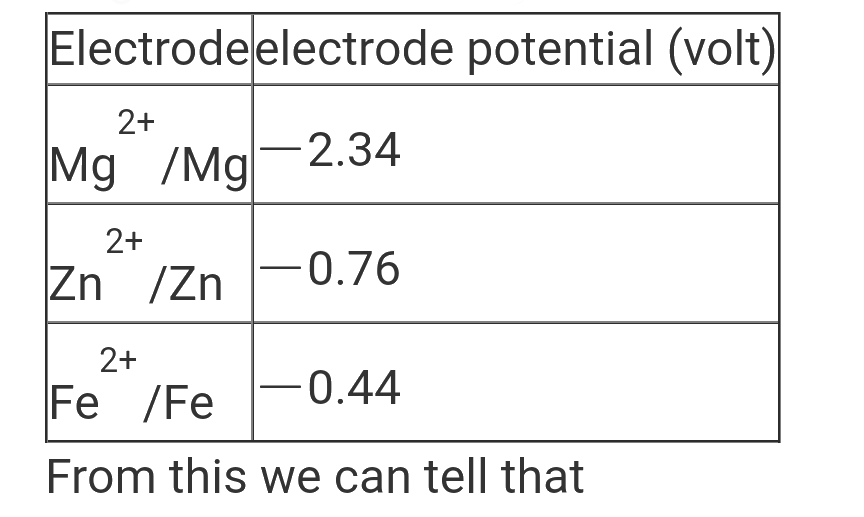
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 67-CHEMISTRY
- An alpha-particle and a proton are accelerated from rest through the s...
Text Solution
|
- The standard reduction potential at 298 K for single electrodes are gi...
Text Solution
|
- Which of the following reaction does not occur?
Text Solution
|
- For the reaction, 2N(2)O(5)to4NO(2)+O(2) rate and rate constant are 1....
Text Solution
|
- Calculate [H^(+)] " and "% dissociation of 0.1 M solution of ammonium ...
Text Solution
|
- A nucleoside on hydrolysis gives
Text Solution
|
- Which one shows the best formation of adduct ?
Text Solution
|
- Which of the following oxyacid contains both P-H and P-P bond simultan...
Text Solution
|
- Which of the following statements are correct for butadiene overset(...
Text Solution
|
- The incorrect statement is
Text Solution
|
- K(p) for the reaction PCl(5)(g)ghArrPCl(3)(g)+Cl(2)(g) at 250^(@)C...
Text Solution
|
- Identify 'A' in the reaction
Text Solution
|
- It is observed that H(2) and He gases always show positive deviation f...
Text Solution
|
Text Solution
|
- The correct order of o^(-) bond lengths in ClO^(-), ClO(2)^(-), ClO(3)...
Text Solution
|
- What sequence of reactions would best accomplish the following convers...
Text Solution
|
- ((dG)/(dp))(T)=
Text Solution
|
- ,
Text Solution
|
- Which of the following compound will not undergo amine inversion?
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|