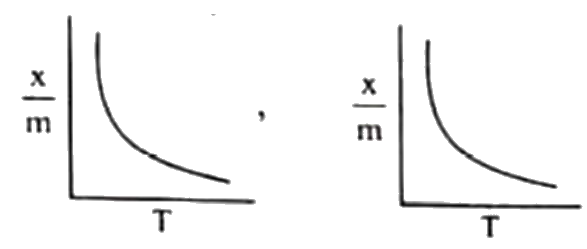
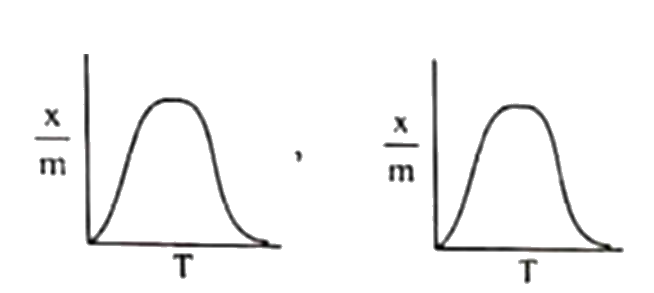
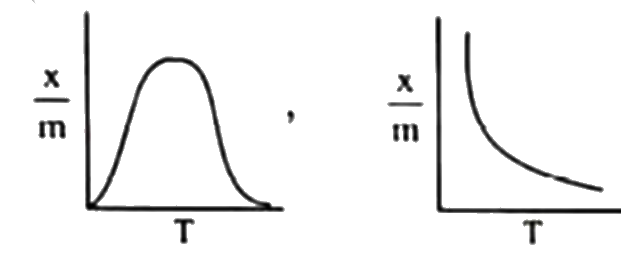
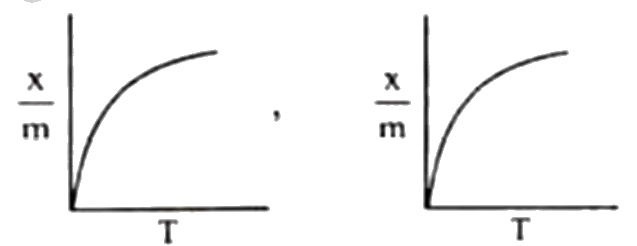
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 60-CHEMISTRY
- The borax bead is due to formation of
Text Solution
|
- A sample of gas contracts by 1 litre against a constant pressure of 0....
Text Solution
|
- An olefinic compound on reductive ozonolysis produces formaldehyde, ac...
Text Solution
|
- Which of the following will be most stable diazonium salt RN2^(+)X^(-)...
Text Solution
|
- Which is not true above white phosphorus?
Text Solution
|
- Alcohol (ROH) does not react with NaBr to form alkyl bromide because
Text Solution
|
- Which is the correct order of ionic sizes ? (Atomic no . : Ce = 58 ,...
Text Solution
|
- Which of the following does not produce any gaseous product when react...
Text Solution
|
- Which of the following is involved in the extraction of Ag from argent...
Text Solution
|
- Choose the correct option for the given structure
Text Solution
|
- Which of the following is not oxidized by aqueous Br2 ?
Text Solution
|
- Select correct adsorption isobars for chemisorption and physisrption r...
Text Solution
|
- (xii) Which is known as 'blister copper' ?
Text Solution
|
- Which of the following sets of quantum numberrs discribes the elecron ...
Text Solution
|
- Which of the following statements are incorrect about phenol formaldeh...
Text Solution
|
- Salt P+ H(2)SO4rarrRoverset(BaCl2)rarr white ppt (P) is paramagnetic ...
Text Solution
|
- The incorrect structure of glycine at given pH are :
Text Solution
|
- In a hydrogen atom, the transition takes place from n = 3 to n = 2 . I...
Text Solution
|
- For the following pattern of hybridization shown by the central atom, ...
Text Solution
|
- Which of the following is known as freon which is used as a refrigeran...
Text Solution
|