A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 72-PHYSICS
- If the temperature of the sun were to increase form T to 2T and its ra...
Text Solution
|
- A Carnot engine working between 300 Kand 400 K has 800 J of useful wor...
Text Solution
|
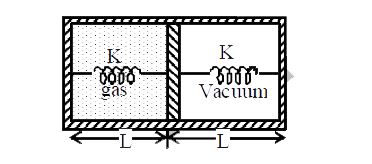
- When heat is supplied to the gas it expands and displaces piston by L/...
Text Solution
|
- A galvanometer with a scale divided into 100 equal divisions has a cur...
Text Solution
|
- An electron (mass =9.1xx10^(-31)kg, charge =1.6xx10^(-19)C) experience...
Text Solution
|
- The real angle of dip, if a magnet is suspended at an angle of 30^(@) ...
Text Solution
|
- The x and y coordinates of a particle at any time t are given by x=7t+...
Text Solution
|
- A large number of bullets are fired in all directions with the same sp...
Text Solution
|
- A body of mass 50 kg is suspended using a spring balance inside a lift...
Text Solution
|
- The normal reaction on a body placed in a lift moving up with constant...
Text Solution
|
- One microgram of matter converted into energy will give.
Text Solution
|
- In a radioactive disintegration, the ratio of initial number of atoms ...
Text Solution
|
- A particle of mass 3 kg , attached to a spring with force constant 48N...
Text Solution
|
- A point performs simple harmonic oscillation of period T and the equat...
Text Solution
|
- Huygen's wave theory of light could not explain
Text Solution
|
- At its closet approach, the distance between the mars and the earth is...
Text Solution
|
- A block of wood floats in freshwater with two - third of its volume su...
Text Solution
|
- An ideal fluid flows through a pipe of circular cross - section with d...
Text Solution
|
- An astronomical telescope has an angular magnification of magnitude 5 ...
Text Solution
|
- A compound microscope having magnifying power 35 with its eye - piece ...
Text Solution
|