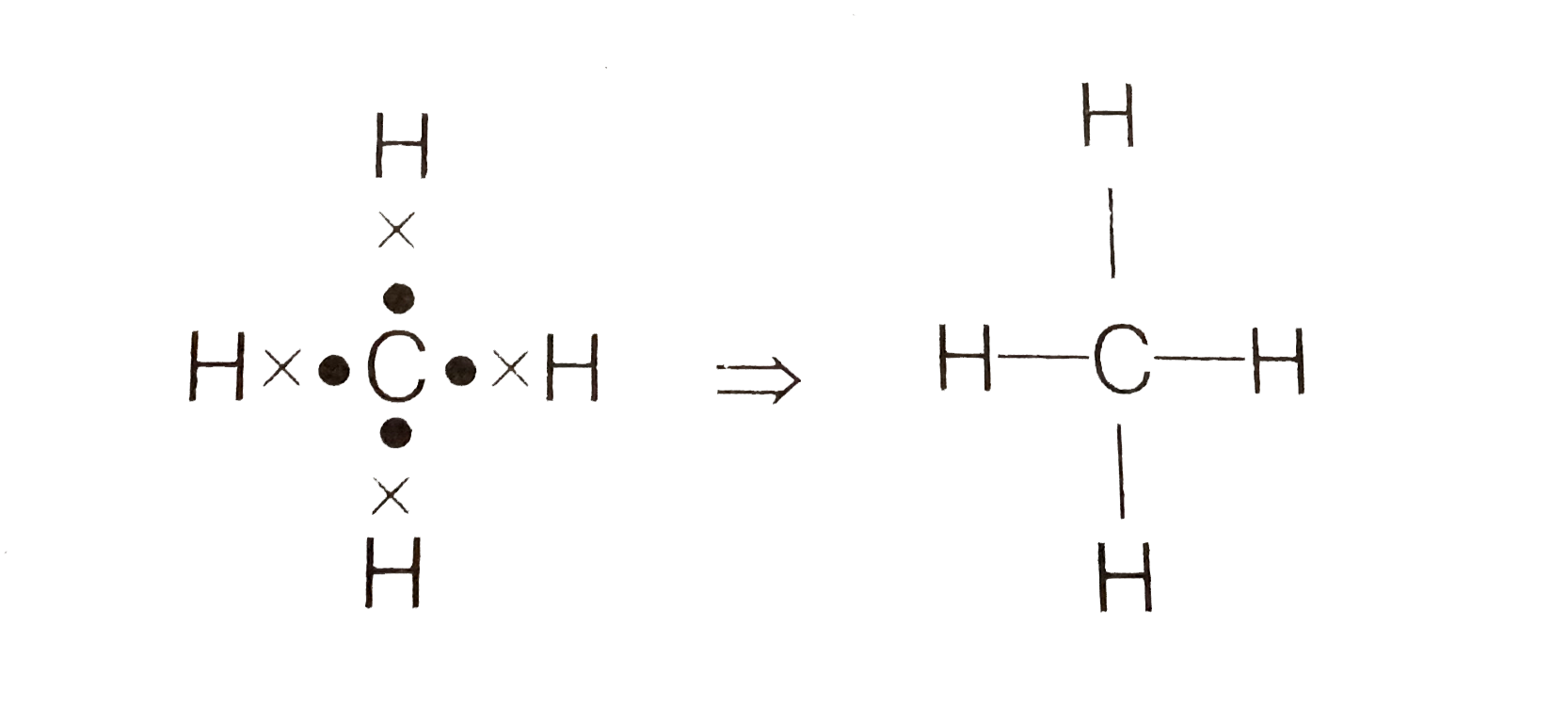A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CARBON AND ITS COMPOUNDS-Carbon And Its Compounds
- Vinegar is a solution of
Text Solution
|
- Mineral acids are stronger acids than carboxylic acids because (i) m...
Text Solution
|
- Carbon forms four covalent bonds by sharing its four valence electrons...
Text Solution
|
- The correct electron dot structure of a water molecule is
Text Solution
|
- Which of the following is not a straight chain hydrocarbon ?
Text Solution
|
- Which amond the following are unsaturated hydrocarbons ? (i) H(3)C-C...
Text Solution
|
- Which of the following does not belong to the same homologous series ?
Text Solution
|
- The name of the compound of CH(3)-CH(2)-CHO ;
Text Solution
|
- The heteroatoms present in CH(3)-CH(2)-O-CH(2)-CH(2)Cl (i) oxygen,...
Text Solution
|
- Which of the following represents saponification reaction ?
Text Solution
|
- The first member of alkyne homologous series is
Text Solution
|
- Draw the electron dot structure of ethyne and also draw its structure ...
Text Solution
|
- Write the names of the following compounds. (a) H-underset(H)unders...
Text Solution
|
- Identify and name the functional groups present in the following compo...
Text Solution
|
- A compounds X is fromed by the reaction of a carboxylic acid C(2)H(4)O...
Text Solution
|
- Why detergents are better cleansing agents than soaps ? Explain.
Text Solution
|
- Name the functional groups present in the following compounds. (a) ...
Text Solution
|
- How is ethene prepared from ethanol ? Give the reaction involved in it...
Text Solution
|
- Intake of small quantity of methanol can be lethal. Comment.
Text Solution
|
- A gas is evolved when ethanol reacts with sodium. Name the gas evolved...
Text Solution
|