Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CARBON AND ITS COMPOUNDS-Carbon And Its Compounds
- A gas is evolved when ethanol reacts with sodium. Name the gas evolved...
Text Solution
|
- Ethene is formed when ethanol at 443 K is heated with excess of concen...
Text Solution
|
- Carbon, group (14) element in the periodic table, is know to from comp...
Text Solution
|
- In electron dot structure, The valence shell electrons are represented...
Text Solution
|
- Catenation is the ability of an atom to form bonds with other atoms of...
Text Solution
|
- Unsaturated hydrocarbons contain multiple bonds between the two C-atom...
Text Solution
|
- Match the reactions given in Column I with the names given in Column I...
Text Solution
|
- Write the structural formulae of all isomers of hexane.
Text Solution
|
- What is the role of metal or reagents written on arrows in the given c...
Text Solution
|
- A salt X is formed and a gas is evolved when ethanoic acid reacts with...
Text Solution
|
- (a) What are hydrocarbons ? Give examples. (b) Give the structural d...
Text Solution
|
- Name the reaction which is commonly used in the conversion of vegetabl...
Text Solution
|
- (a) Write the formula and draw electron dot structure of carbon tetra...
Text Solution
|
- Esters are sweet-smelling substances and are used in making perfumes. ...
Text Solution
|
- A compound C (molecular formula, C(2)H(4)O(2)) reacts with Na metal to...
Text Solution
|
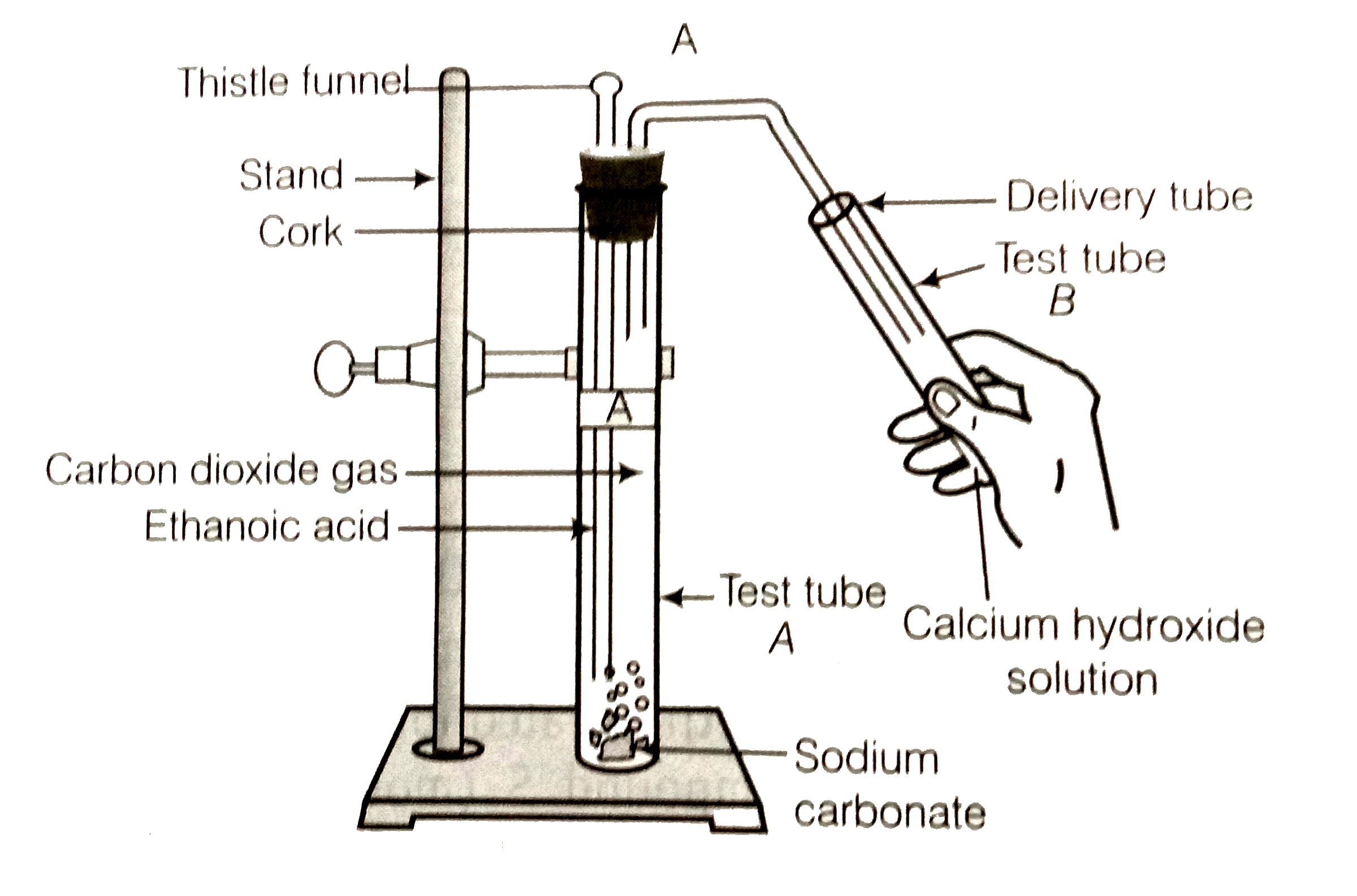
- Look at the figure and answer the following questions. (a) What...
Text Solution
|
- How would you bring about the following conversions ? Name the process...
Text Solution
|
- Draw the possible isomers of the compound with molecular formula C(3)H...
Text Solution
|
- Explain the given reactions with the examples. (a) Hydrogenation rea...
Text Solution
|
- An organic compound A on heating with concentrated H(2)SO(4) forms a c...
Text Solution
|