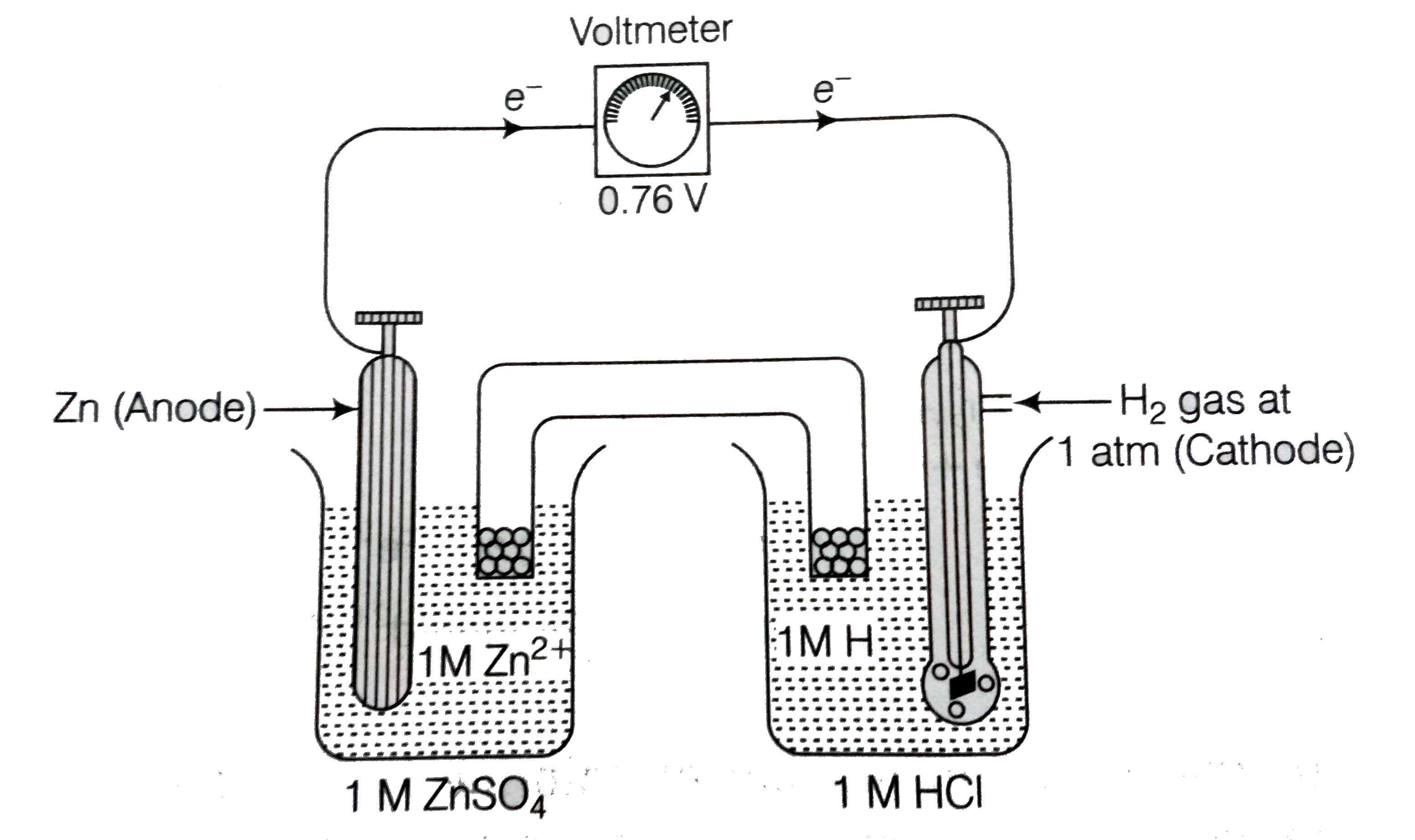
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-REDOX REACTIONS-LONG ANSWER TYPE QUESTIONS
- Explain redox reaction on the basis of electron transfer, Given suitab...
Text Solution
|
- On the basis of standard electrode potential values, suggest which of ...
Text Solution
|
- Which of the following elements does not show disproportionation tende...
Text Solution
|
- Write redox couples involved in the reactions (a) to (d) given in que...
Text Solution
|
- Find out the oxidation number of chlorine in the following compounds a...
Text Solution
|
- Which method can be used to find out strength of reductant/oxidant in ...
Text Solution
|