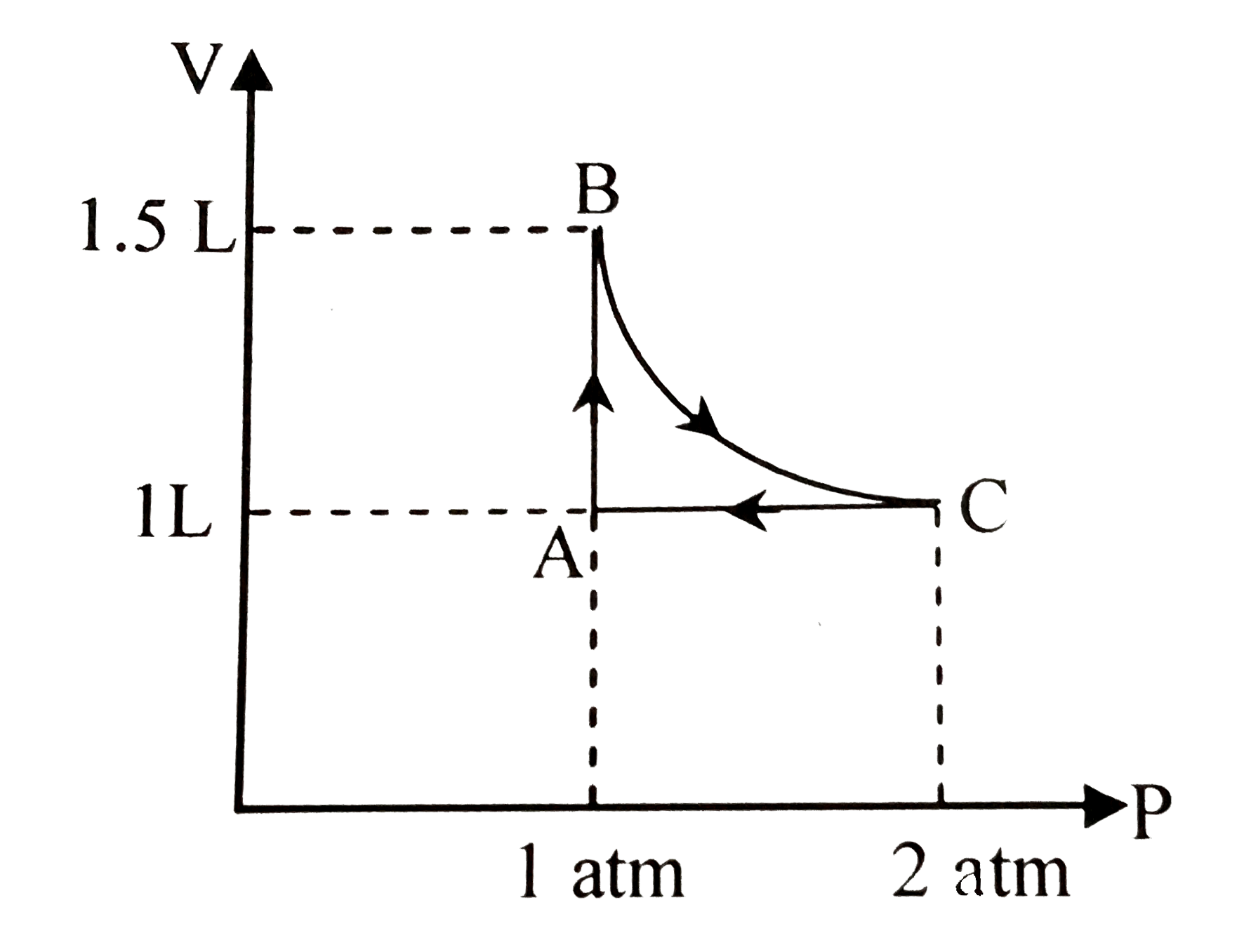
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-KVPY-Part B- Chemistry
- In the following reaction squence X and Y are
Text Solution
|
- In the following reactions X and Y are
Text Solution
|
- Which of the following alkenes can generate optically active compounds...
Text Solution
|
- When heated in air, brown copper powder turns black. This black powder...
Text Solution
|
- The geometry and magnetic property of [NiCl(4)]^(2-), respectively are...
Text Solution
|
- Among (i) [Cr(en)(3)]^(3+), (ii) trans - [Cr(en)(2)Cl(2)]^(+), (iii) C...
Text Solution
|
- ""^(227)Ac has a half-life of 22 years with respect to radioactive dec...
Text Solution
|
- A system consisting of 1 mol of an ideal gas undergoes a reversible pr...
Text Solution
|
- A mixture of toluene and benzene boils at 100^(@)C. Assuming ideal beh...
Text Solution
|
- A two-dimensional solid pattern formed by two different atoms X and Y ...
Text Solution
|