A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-KVPY-Part 2 Chemistry
- Among the following, the species with identical bond order are
Text Solution
|
- The quantity of heat (in J) required to raise temperature of 1.0 kg of...
Text Solution
|
- A solution of 20.2 g of 1,2-dibromopropane in MeOH upon heating with e...
Text Solution
|
- The lowest stability of ethyl anion compared to methyl anion and the h...
Text Solution
|
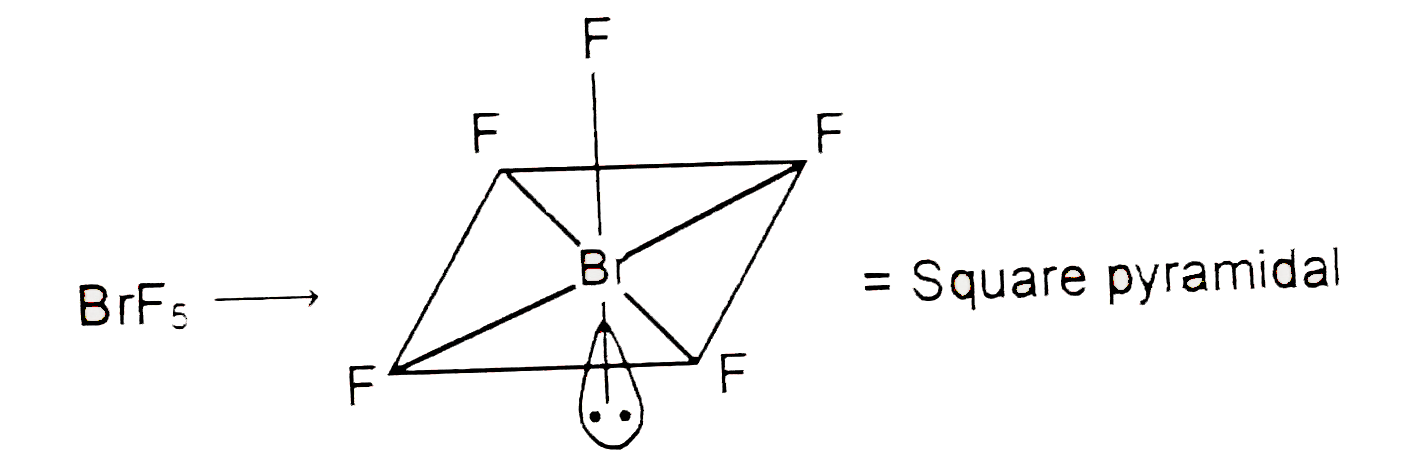
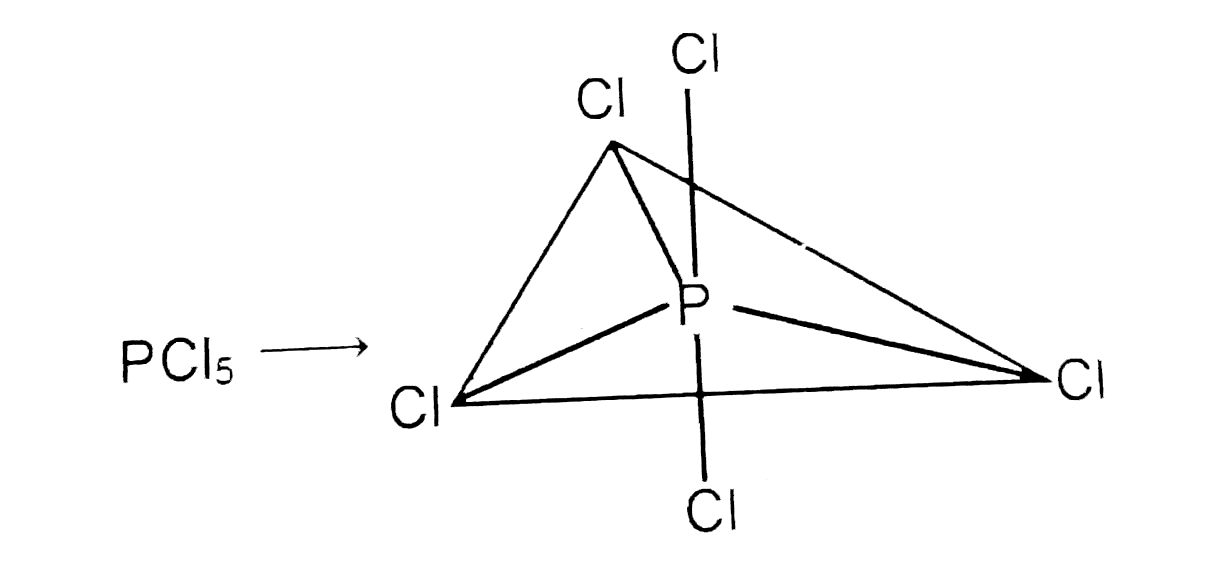
- The F-B -F bond angels in BrF(5)" and " Cl-P-Cl bond angls in PCl(5), ...
Text Solution
|
- 10 moles of a mixture of hydrogen and oxygen gases at a pressure of 1 ...
Text Solution
|
- The ammonia evolved from 2 g of a compound in Kjeldahl's estimation of...
Text Solution
|
- Compelete reaction of 2.0 g calcium (at wt. = 40) with excess HCl prod...
Text Solution
|
- A compound X formed after heating coke with lime react with water to g...
Text Solution
|
- In the following reaction sequence X and Y are, respectively
Text Solution
|
- Among the following compouds, E/Z isomerism is possible for
Text Solution
|
- In the reaction x and y, respectively, are
Text Solution
|
- Among the following molecules, the one with the largest bond angle at ...
Text Solution
|
- A compound has the following composition by weight , Na = 18.60 %, S =...
Text Solution
|
- X g of ice at 0 .^(@)C is added to 340 g of water at 20^(@)C. The fina...
Text Solution
|