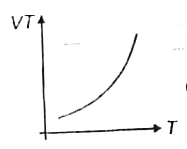
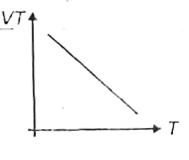
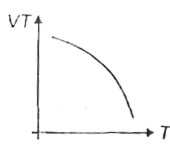
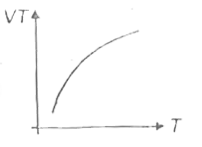
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY
AAKASH INSTITUTE|Exercise EXERCISE (ASSIGNMENT) SECTION - C Previous Years Questions|21 VideosKINETIC THEORY
AAKASH INSTITUTE|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|10 VideosKINETIC THEORY
AAKASH INSTITUTE|Exercise EXERCISE (ASSIGNMENT) SECTION - A Objective Type Questions|30 VideosGRAVITATION
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION - D (ASSERTION-REASON TYPE QUESTIONS)|16 VideosLAWS OF MOTION
AAKASH INSTITUTE|Exercise Assignment (SECTION-D) (Assertion-Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-KINETIC THEORY-EXERCISE (ASSIGNMENT) SECTION - B Objective Type Questions
- A container contains 32 g of O2 at a temperature T. The pressure of th...
Text Solution
|
- An ideal gas is expanding such that PT^2=constant. The coefficient of ...
Text Solution
|
- 50 cal of heat is required to raise the temperature of 1 mole of an id...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is shown in figure. ...
Text Solution
|
- The energy (in eV) possessed by a neon atom at 27^@ C is
Text Solution
|
- If heat energy is given to an ideal gas at constant pressure, then se...
Text Solution
|
- If hydrogen gas is heated to a very high temperature, then the fractio...
Text Solution
|
- The temperature (T) of one mole of an ideal gas varies with its volume...
Text Solution
|
- Nitrogen gas is filled in an isolated container. If alpha fraction of ...
Text Solution
|
- An ideal has undergoes a polytropic given by equation PV^(n) = constan...
Text Solution
|
- If alpha moles of a monoatomic gas are mixed with beta moles of a poly...
Text Solution
|
- If different ideal gases are at the same temperature, pressure and hav...
Text Solution
|
- The internal energy of 10 g of nitrogen at N. T. P. is about
Text Solution
|
- The mean free path of a molecule of He gas is alpha. Its mean free pat...
Text Solution
|
- According to C.E. van der Waal, the interatomic potential varies with ...
Text Solution
|
- The value of critical temperature in terms of van der Waals' constants...
Text Solution
|
- To find out degree of freedom, the correct expression is:
Text Solution
|
- Nitrogen gas N2 of mass 28 g is kept in a vessel at pressure of 10 atm...
Text Solution
|
- A diatomic gas of molecular mass 40 g/mol is filled in rigid container...
Text Solution
|
- The ratio of average translatory kinetic energy of He has molecules to...
Text Solution
|