A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
OP TANDON|Exercise Set-2|46 VideosATOMIC STRUCTURE
OP TANDON|Exercise Objective Question Level-B|51 VideosATOMIC STRUCTURE
OP TANDON|Exercise Practice Problems|88 VideosAROMATIC HYDROCARBONS (ARENES)
OP TANDON|Exercise EXAMPLES|24 VideosCHARACTERISATION OF ORGANIC COMPOUNDS
OP TANDON|Exercise Passage-2|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ATOMIC STRUCTURE -Questions with single correct Answer
- In any subshell, the maimum number of electrons having same value of s...
Text Solution
|
- Values of magnetic orbital quantum for an electron of M-shell can be:
Text Solution
|
- The correct set of quantum number for the unpaired electron of a chlor...
Text Solution
|
- In hydrogen atom, the electron is at a distance of 4.768Ã… from the nu...
Text Solution
|
- The total number of m values for n = 4 is
Text Solution
|
- The subshell that rises after f subshell is called g subshell What i...
Text Solution
|
- In Bohr's model, if the atomic radius of the first orbit is r(0), then...
Text Solution
|
- The electrons identified by quantum numbers n and l :- (a) n=4, l=1 ...
Text Solution
|
- The angular momentum of an electron depends on:
Text Solution
|
- The correct set of quantum number for the unpaired electron of a chlor...
Text Solution
|
- The shape of the orbital is determined by
Text Solution
|
- The energy of an electron of 2p(y) orbital is
Text Solution
|
- Two electrons occupying the same orbital are distinguished by :
Text Solution
|
- The maximum number of electrons in a subshell is given by the expressi...
Text Solution
|
- The electronic configuration of an atom/ion can be defined by the foll...
Text Solution
|
- If an electron has spin quantum number of -(1)/(2) and magnetic quant...
Text Solution
|
- For the energy level is an atom which one of the following statement i...
Text Solution
|
- A new electron enters the orbital when:
Text Solution
|
- For a given value of n (principal quantum number), the energy of diffe...
Text Solution
|
- After filling the 4d-orbitals, an electron will enter in:
Text Solution
|
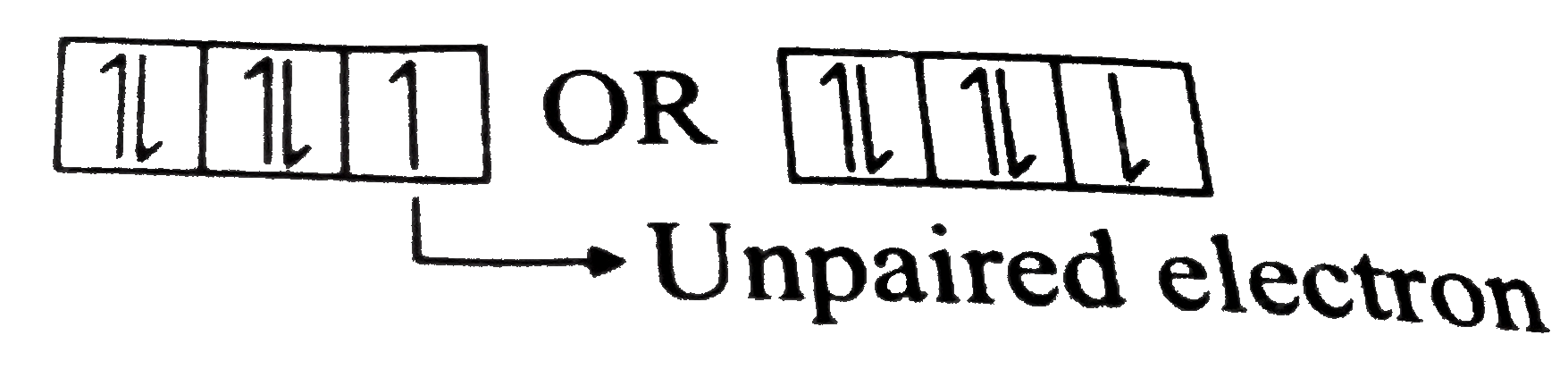 ltBrgt `Cl_(17)to1s^(2),2s^(2)2p^(6),3s^(2)3p^(5)`
ltBrgt `Cl_(17)to1s^(2),2s^(2)2p^(6),3s^(2)3p^(5)`