A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
OP TANDON|Exercise Set-2|46 VideosATOMIC STRUCTURE
OP TANDON|Exercise Objective Question Level-B|51 VideosATOMIC STRUCTURE
OP TANDON|Exercise Practice Problems|88 VideosAROMATIC HYDROCARBONS (ARENES)
OP TANDON|Exercise EXAMPLES|24 VideosCHARACTERISATION OF ORGANIC COMPOUNDS
OP TANDON|Exercise Passage-2|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ATOMIC STRUCTURE -Questions with single correct Answer
- Photo electric effect can be explained only by assuming that light
Text Solution
|
- Photoelectric effect supports quantum nature of light because (a) th...
Text Solution
|
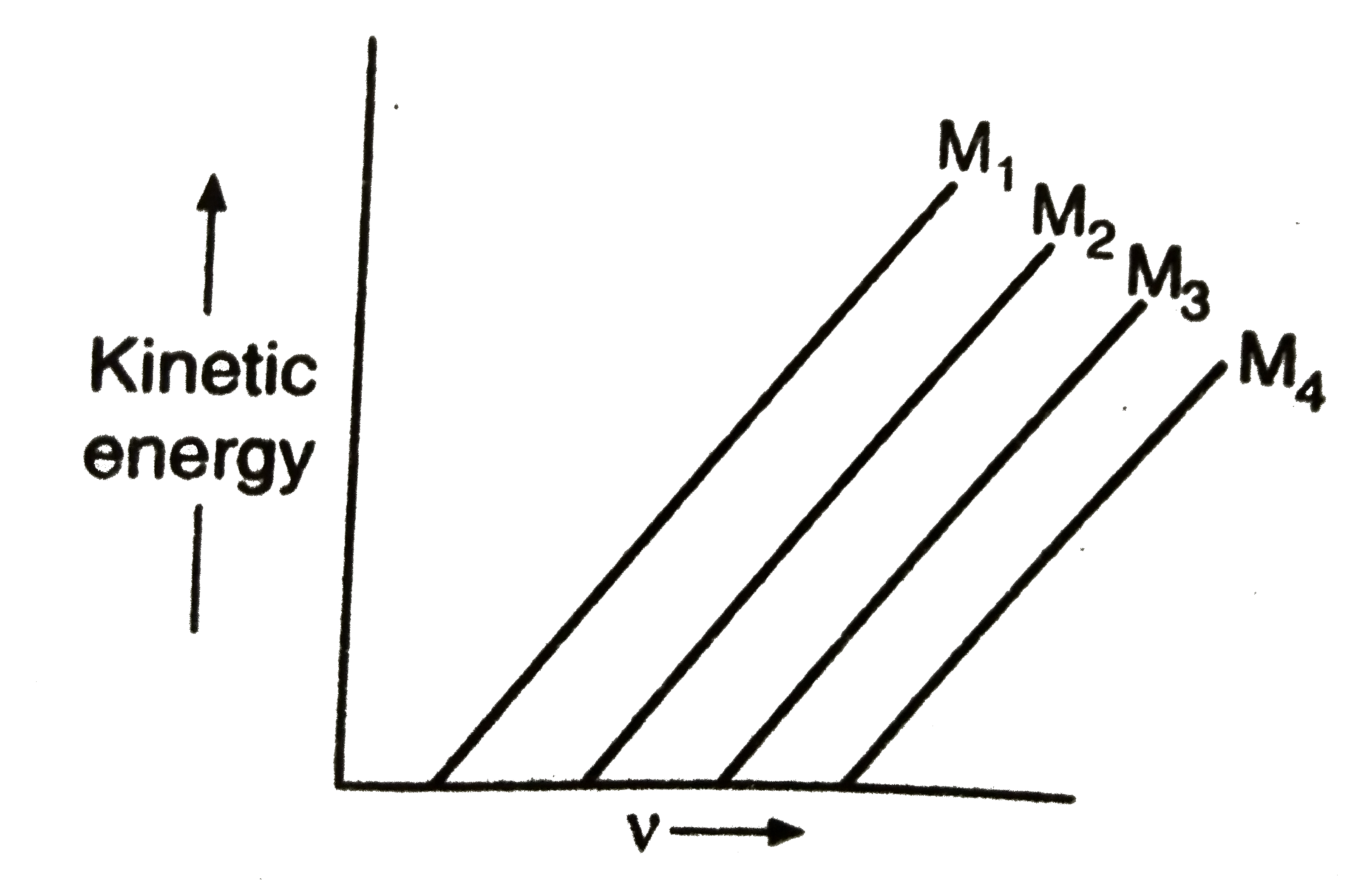
- A plot of the kinetic energy ((1)/(2)mv^(2)) of ejected electrons as a...
Text Solution
|
- Momentum of a photon of wavelength lamda is
Text Solution
|
- When X-rays pass through air they:
Text Solution
|
- X-rays
Text Solution
|
- The energy of an electron in the first Bohr orbit of H atom is -13.6 e...
Text Solution
|
- The electrons identified by the following quantum numbers n and l: (i)...
Text Solution
|
- The wavelength of the radiation emitted when an electron falls from Bo...
Text Solution
|
- The energy of the electron in the first orbit of He^+ is - 871.6 xx 10...
Text Solution
|
- The wavelength associated with a holf ball weighing 200 g and moving w...
Text Solution
|
- If uncertainty in momentum of electron is three times the uncertainty ...
Text Solution
|
- Threshold wavelength of a metal is lamda(0). The de Broglie wavelength...
Text Solution
|
- The number of nodal planes in p(x)-obital is:
Text Solution
|
- The angular momentum (L) of an electron in a Bohr orbit is gives as:
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- Which of the following statement(s) are correct? 1. Electronic confi...
Text Solution
|
- The electronic configuration of an element is 1s^(2)2s^(2)2p^(6)3s^(2)...
Text Solution
|
- The quantum number + 1//2 and -1//2 for the electron spin represent
Text Solution
|
- Rutherford's scattering experiment, which established the nuclear mode...
Text Solution
|