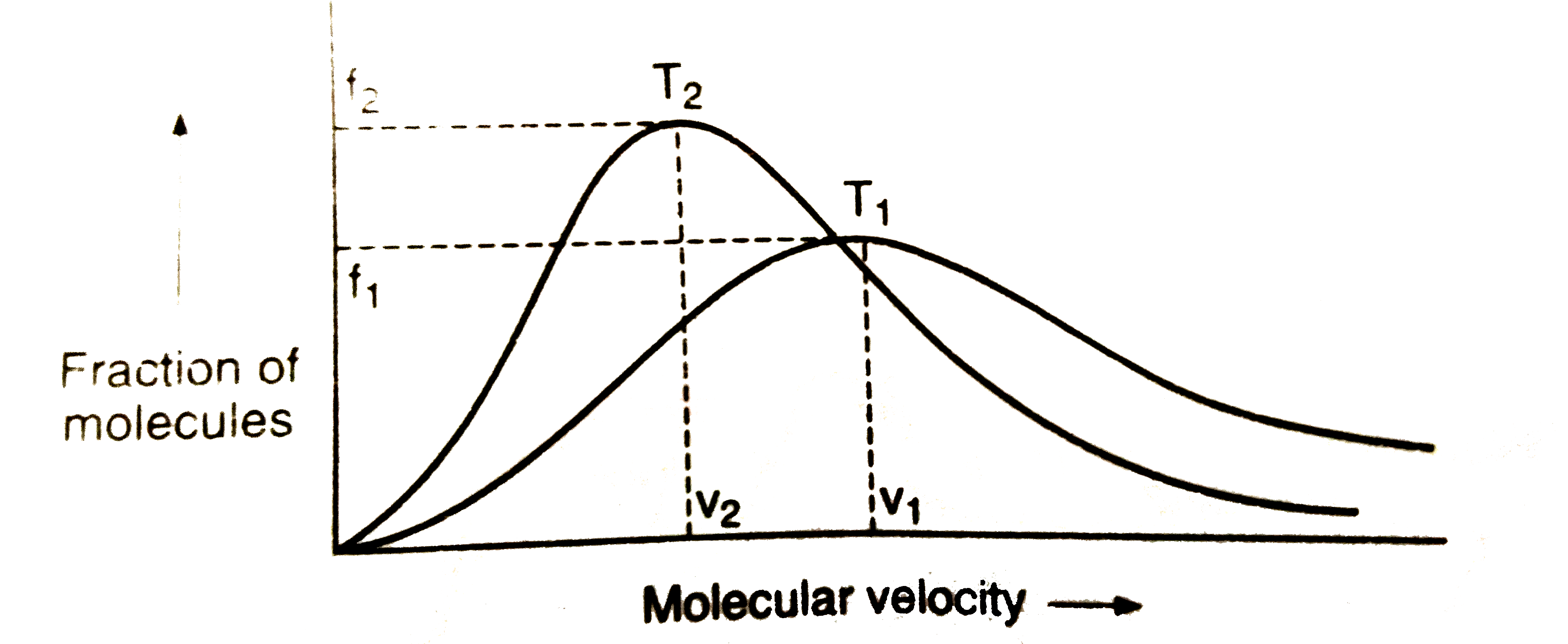
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Practice Problems|53 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Objective questions|105 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-Illustration
- Under similar conditions which of the following gases will diffuse fou...
Text Solution
|
- The rates of diffusion of hydrogen and deuterium are in the ratio:
Text Solution
|
- The time taken for effusion of 64 mL of oxygen will be as the time tak...
Text Solution
|
- Which of the following pairs of gses will have identical rate of effus...
Text Solution
|
- Two gases bulbs A and B are connected by a tube having a stopcock. Bu...
Text Solution
|
- At what temperature root mean sqaure of N(2) gas is equal to that of p...
Text Solution
|
- At what temperature is the kinetic energy of a gas molecule half of it...
Text Solution
|
- The root mean square speed fo molecules of nitrogen gas is v at a cert...
Text Solution
|
- Plot of Maxwell's distribution of velocities is given below: whic...
Text Solution
|
- At what temperature is the rms speed of H(2) molecules the same as tha...
Text Solution
|
- In the temperature of 1 mole of a gas is increased by 50^(@)C. Calcula...
Text Solution
|
- At same temperature, calculate the ratio of average velocity of SO(2) ...
Text Solution
|
- If the most probable velocity of methane at a certain temperature is 4...
Text Solution
|
- At high temperature and low pressure the van der Waals equation is red...
Text Solution
|
- The constant 'a' in van der Waal's equaton is maximum in:
Text Solution
|
- A gas described by van der Waal's equation:
Text Solution
|
- van der Waal's constants of two gases X and Y are as given: {:(,a ("...
Text Solution
|
- Select the correct statement about van der Waal's constant 'b' 1. I...
Text Solution
|
- Gases X, Y, Z, P and Q have the van der Waal's constants 'a' and 'b' (...
Text Solution
|
- At high pressure, van der Waal's equation becomes:
Text Solution
|