A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-Self Assess,ent
- The ratio of Boyle's temperature and critical temperature for a gas is...
Text Solution
|
- Kinetic energy and pressure of a gas of unit mole are related as :
Text Solution
|
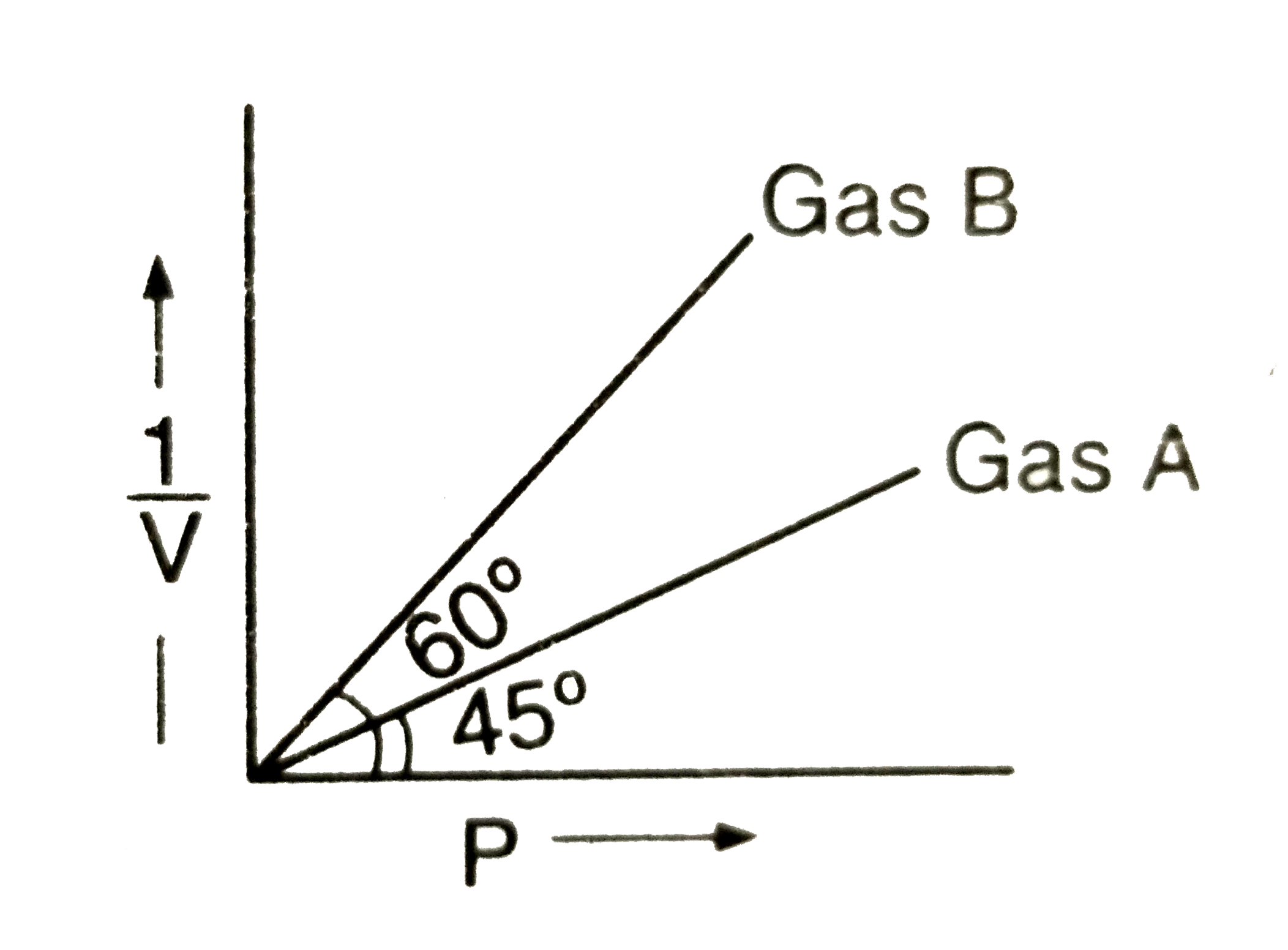
- At constant temperature of 273 K, (1)/(V) vs P are plotted for two ide...
Text Solution
|
- At low pressure, the graph of PV vs (1)/(V) for a given amount at a co...
Text Solution
|
- Which of the following measure the deviation from ideal behaviour of g...
Text Solution
|
- Which of the following mixtures of gases at room temperature follow Da...
Text Solution
|
- A real gas can be liquefied:
Text Solution
|
- In the equation PV = nRT, the value of 'R' will not depend on:
Text Solution
|
- Which of the following processes would lead to an increase in the aver...
Text Solution
|
- Statement-I : The pressure inside the LPG cylinder remains constant ev...
Text Solution
|
- Statement-I : If a gas has compressilbility factor (Z) greater than un...
Text Solution
|
- Statement-I : The value of Boyle's temperature for a real gas is (T(B...
Text Solution
|
- Statement-I : A gas effuses our from a flask through an orifice (pinho...
Text Solution
|
- Statement-I : van der Waals' constant 'a' depends on the intermolecula...
Text Solution
|
- This section contains 2 questions. Each question contains statement gi...
Text Solution
|
- This section contains 2 questions. Each question contains statement gi...
Text Solution
|
- Compressibility factor for ideal gas will be equal to ...
Text Solution
|
- 400 mL of ammonia gas at a pressure of 300 mm and 200 mL of hydrogen c...
Text Solution
|
- A mixture of 2 g hydrogen and 64 g oxygen exerts a pressure of 3 atm. ...
Text Solution
|
- Air enters in the lungs in a tiny cavity called alveoli. Oxygen diffus...
Text Solution
|