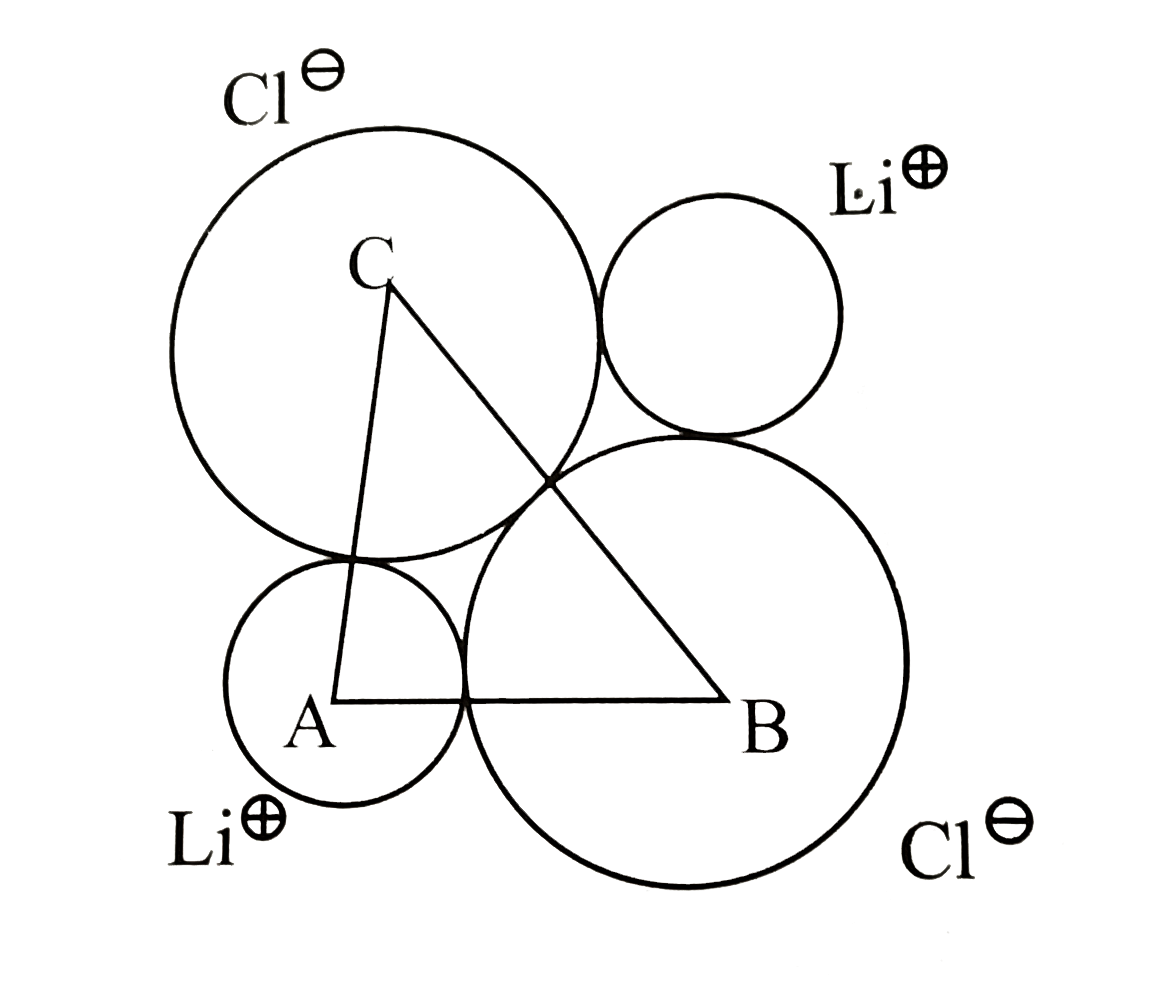
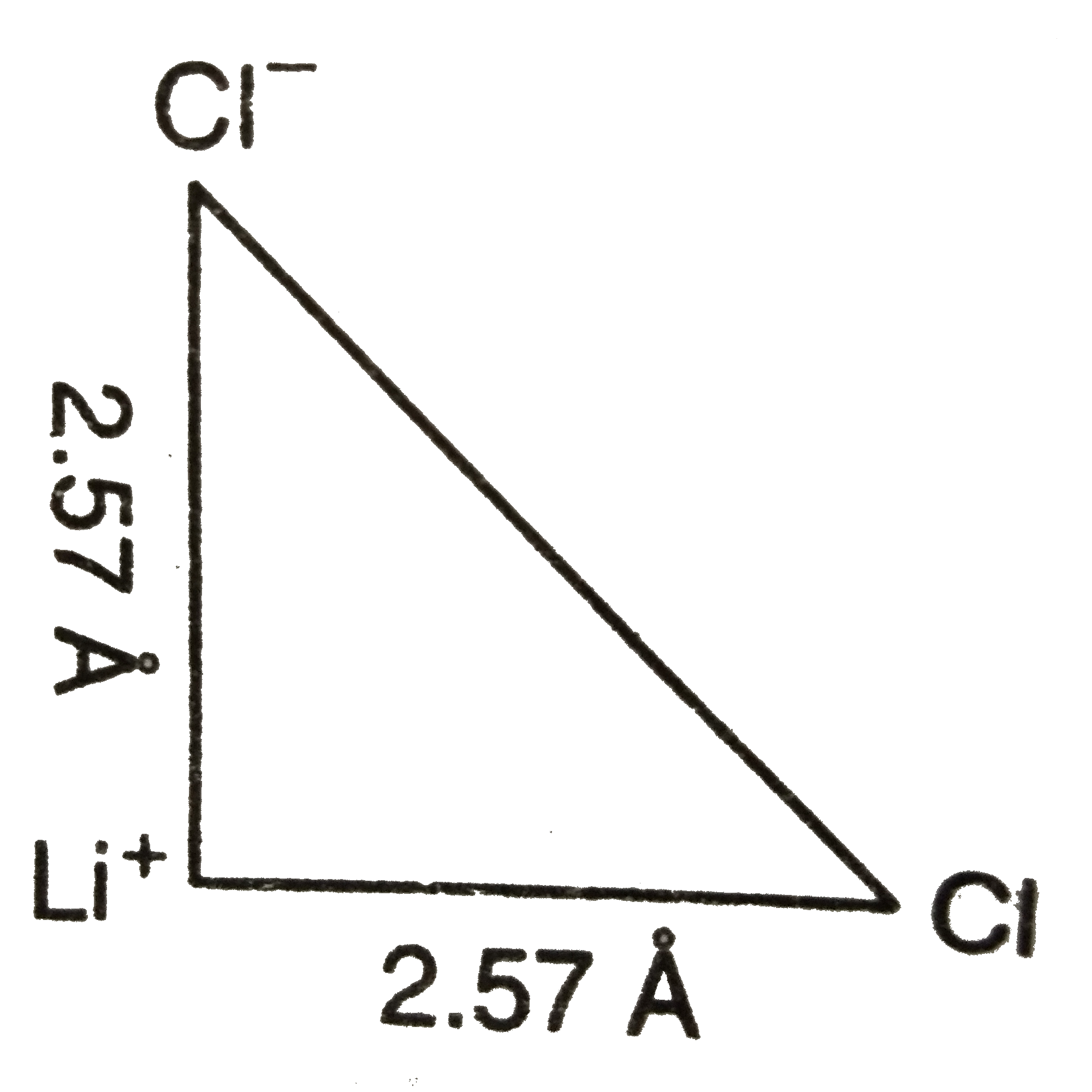Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Illustrations of Objective Questions|35 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Practice Problems|20 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 VideosTHE COLLOIDAL STATE
OP TANDON|Exercise Self Assessment|21 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (SOLID STATE)-SELF ASSESSMENT Section VI
- The unit cube length for LiCl (NaCl structure) is 5.14 Å. Assuming ani...
Text Solution
|
- The closed packed structure have both octahedral and tetrahedral voids...
Text Solution
|
- The closed packed structure have both octahedral and tetrahedral voids...
Text Solution
|
- The closed packed structure have both octahedral and tetrahedral voids...
Text Solution
|