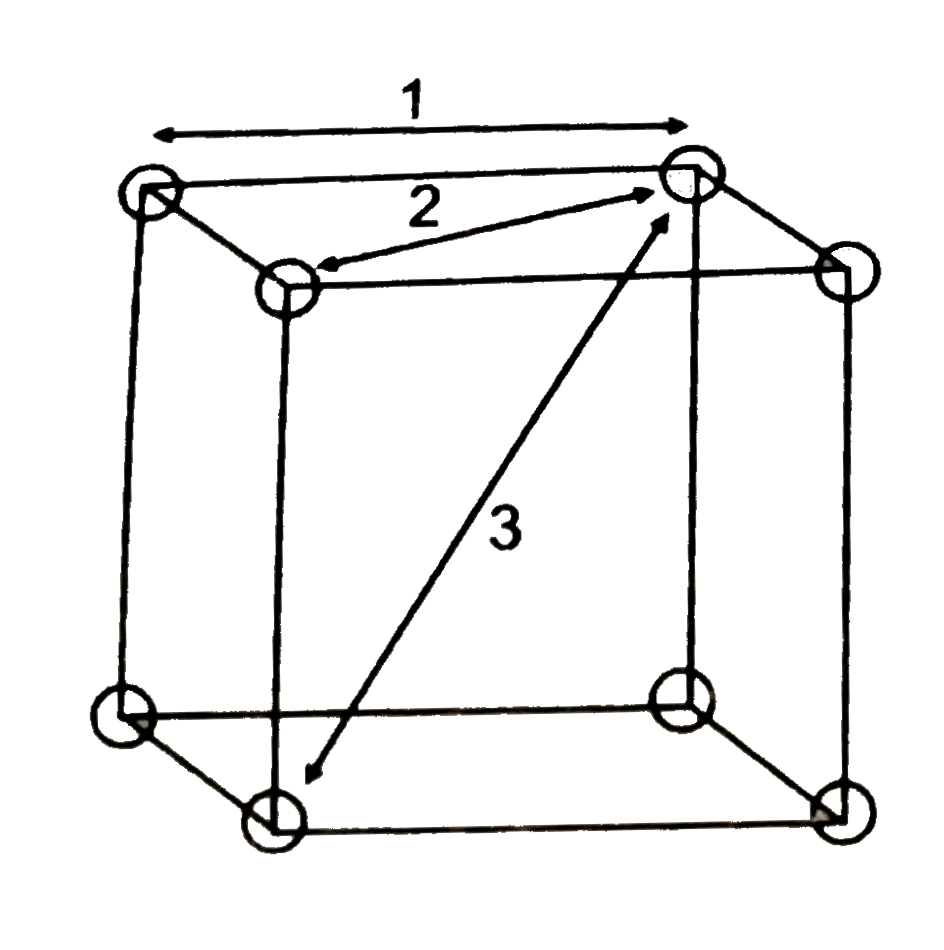
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Practice Problems|20 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL -A)|77 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 VideosTHE COLLOIDAL STATE
OP TANDON|Exercise Self Assessment|21 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (SOLID STATE)-Illustrations of Objective Questions
- Which of the following network solids is an exceptionally good conduct...
Text Solution
|
- Which of the following is not a molecular solid ?
Text Solution
|
- First three nearest neighboure distance for primitive cubic lattice ...
Text Solution
|
- In the fcc arrangement of A and B atoms whose A atoms are at corners o...
Text Solution
|
- A match box exbibits
Text Solution
|
- A solid has a structure in which W atoms are located at the corners of...
Text Solution
|
- An element 'A' has face-centered cubic structure with edge length equa...
Text Solution
|
- The packing fraction of the element that crystallizes in simple cubic ...
Text Solution
|
- Metallic gold crystallises is fcc lattice .The length of the cubic uni...
Text Solution
|
- Sodium metal exits in bcc unit cell .The distance between nearest sodi...
Text Solution
|
- A solid AB has CsCl-type structure. The edge length of the unit cell i...
Text Solution
|
- The interionic distance for cesium chloride crystal will be
Text Solution
|
- If the side length of a face centered unit cell of a metal is 400 pm, ...
Text Solution
|
- In a solid ,oxide (O^(2-)) ions are arranged in ccp, cations (A^(3+)...
Text Solution
|
- In a compound ,atoms of element Y from ccp lattice and those of elemen...
Text Solution
|
- A solid compound contains X,Y and Z atoms in a cubic lattice with X at...
Text Solution
|
- In a crystalline solid,atoms of X from fcc packing and the atoms of Y ...
Text Solution
|
- A solid AB has NaCl structure. If the radius of the cation A is 100 pm...
Text Solution
|
- If the radius of Br^(-) ion is 0.182 nn, how large can a cation be fit...
Text Solution
|
- How many unit cells are present in 39 g of potassium that crystallises...
Text Solution
|