A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Asertion-Reason type Questions|7 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Matrix Matching Type Questions|5 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL -A)|77 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 VideosTHE COLLOIDAL STATE
OP TANDON|Exercise Self Assessment|21 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (SOLID STATE)-Objective Questions (Level-B)
- If the anions (A) form hexagonal closest packing and cations (C ) occu...
Text Solution
|
- In the fcc arrangement of A and B atoms whose A atoms are at corners o...
Text Solution
|
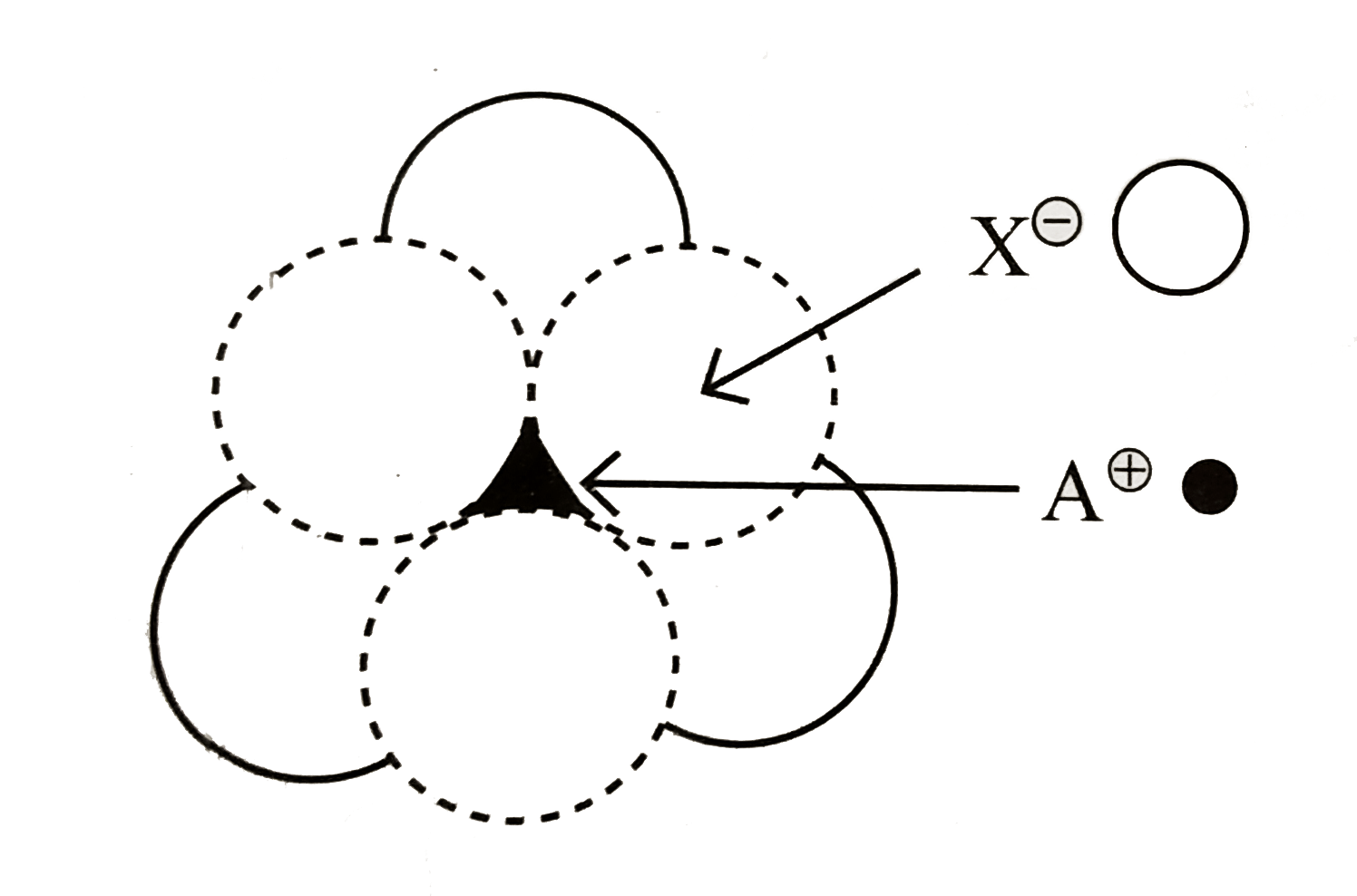
- The arrangement of X^(ɵ) ions around A^(o+) ion in solid AX is given i...
Text Solution
|
- Select the incorrect statement:
Text Solution
|
- The ionic radii of Rb^(+) and I^(-) are 1.46 and 2.16Å. The most proba...
Text Solution
|
- Perovskite is a mineral with the formula CaTiO(3) . Which of the posit...
Text Solution
|
- The number of atoms in 100 g an fcc crystal with density d = 10 g//cm^...
Text Solution
|
- In a ccp structure, the :
Text Solution
|
- Which of the following compounds represents an inverse 2:3 spinel stru...
Text Solution
|
- A solid solution of CdBr(2) in AgBr contains
Text Solution
|
- A crystalline solid is made of X, Y and Z elements. Atoms of X form fc...
Text Solution
|
- Which of the following patterns has voids fraction of 0.26?
Text Solution
|
- In face centred cubic unit cell, what fraction of edge is not covered ...
Text Solution
|
- First three nearest neighbour distance for body centred cubic lattice ...
Text Solution
|
- How many tetrahedral holes are occupied in diamond ?
Text Solution
|
- If the unit cell of a mineral has cubic close packed (ccp) array of ox...
Text Solution
|
- Which of the following crystals have 6:6 coordination ?
Text Solution
|
- Which of the following compounds represents an inverse 2:3 spinel stru...
Text Solution
|
- Which type of crystals contains only one Bravais lattice ?
Text Solution
|
- At which temperature , is a ferrimagnetic solid converted to a ferroma...
Text Solution
|