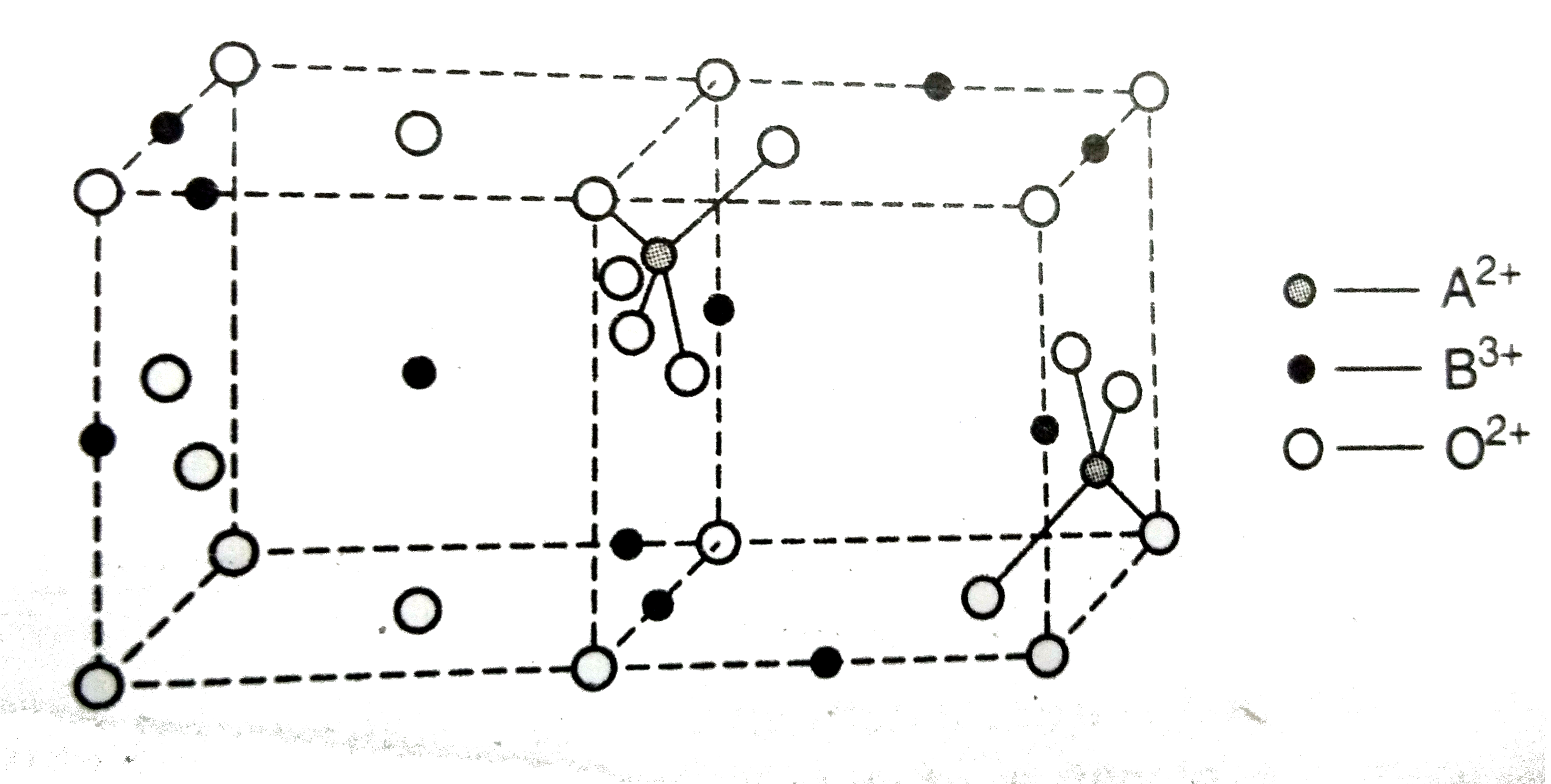
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section I|10 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section II|4 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Integer Answer Type Questions|9 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 VideosTHE COLLOIDAL STATE
OP TANDON|Exercise Self Assessment|21 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (SOLID STATE)-Linked Comprehension Type Questions
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- Packing refers to the arrangement of constituent units in such a way t...
Text Solution
|
- The space lattice given in the figure to :
Text Solution
|
- O(2-) ions are present in :
Text Solution
|
- The formula of the compound is :
Text Solution
|
- Fraction of the total octahedral voids occupied will be :
Text Solution
|
- B^(3+) and A^(2+) ions are present in:
Text Solution
|