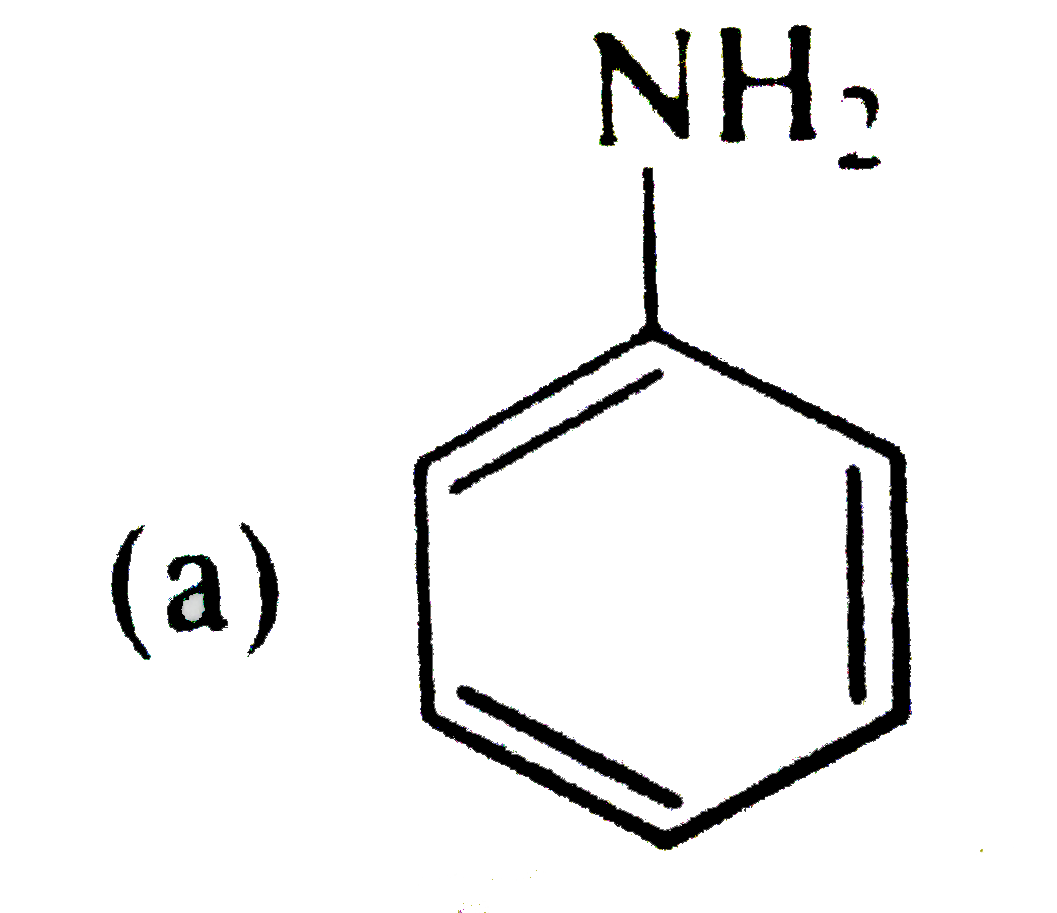
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INTRODUCTION TO ORGANIC CHEMISTRY
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE|4 VideosINTRODUCTION TO ORGANIC CHEMISTRY
OP TANDON|Exercise Passage-3|4 VideosCHEMISTRY IN EVERYDAY LIFE
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 VideosMISCELLANEOUS (TOPICS OF GENERAL INTEREST)
OP TANDON|Exercise SET-VI: Problems on graphical aptitude|53 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-INTRODUCTION TO ORGANIC CHEMISTRY -Passage-4
- The hydrogen bond is an electrostatic attractive force between covalen...
Text Solution
|
- The hydrogen bond is an electrostatic attractive force between covalen...
Text Solution
|
- The hydrogen bond is an electrostatic attractive force between covalen...
Text Solution
|
- The hydrogen bond is an electrostatic attractive force between covalen...
Text Solution
|
- The hydrogen bond is an electrostatic attractive force between covalen...
Text Solution
|