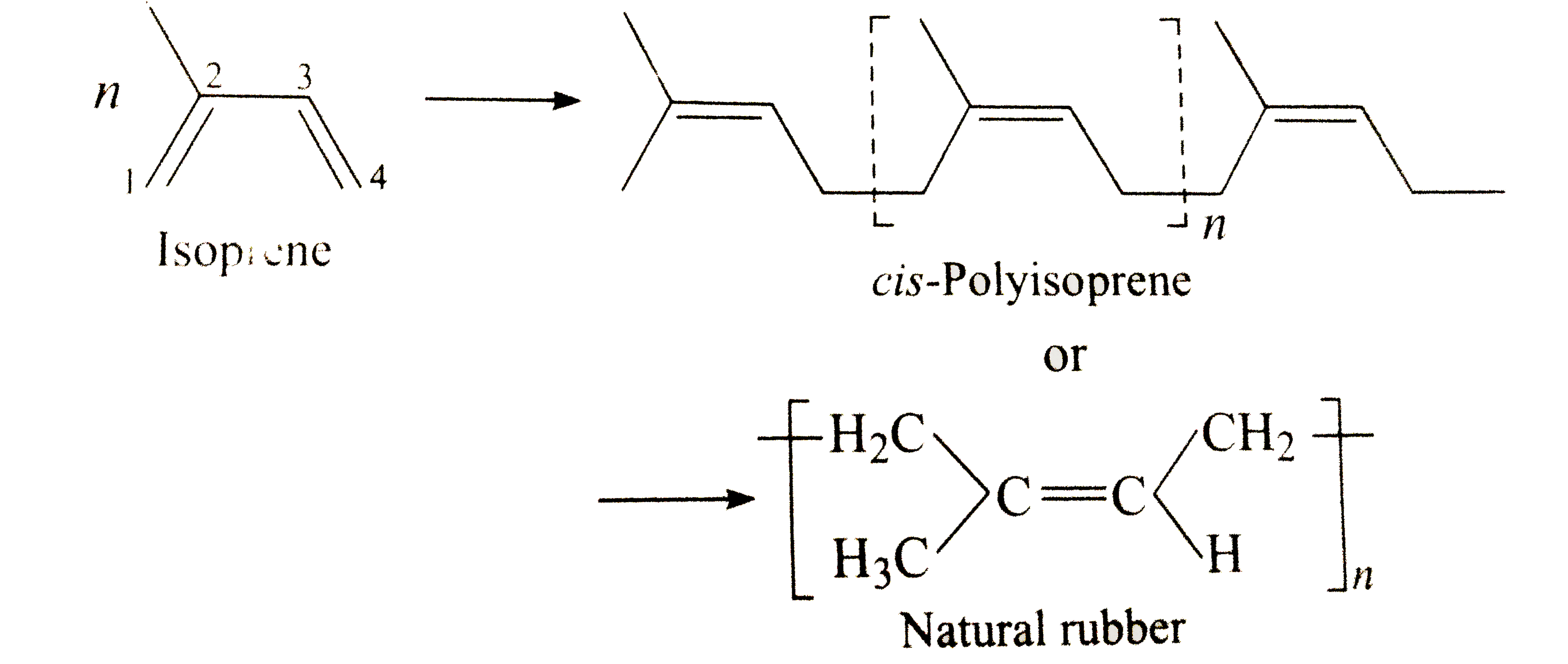Text Solution
Verified by Experts
Topper's Solved these Questions
POLYMERS
OP TANDON|Exercise LEVEL A|1 VideosPOLYMERS
OP TANDON|Exercise SET II|1 VideosPOLYMERS
OP TANDON|Exercise PASSAGE 3|5 VideosOXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise SECTION V|3 VideosPROBLEMS BASED UPON STRUCTURES AND REACTIONS OF ORGANIC COMPOUNDS
OP TANDON|Exercise Some Solved Problems|21 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-POLYMERS-PROBLEMS FOR PRACTISE
- Write the structures of the monomers of the following polymers: (i) ...
Text Solution
|
- How will you synthesise? (a) Polyvinyl chloride (PVC) from acetylene
Text Solution
|
- Describe how the following polymers are synthesised? (i) Nylon-6,6 (...
Text Solution
|
- Write the free radical mechanism for the polymersation of ethene.
Text Solution
|
- How does the presence of benzoquinone inhibit the free radical polymer...
Text Solution
|
- How does the presence of double bonds in rubber molecules influence th...
Text Solution
|