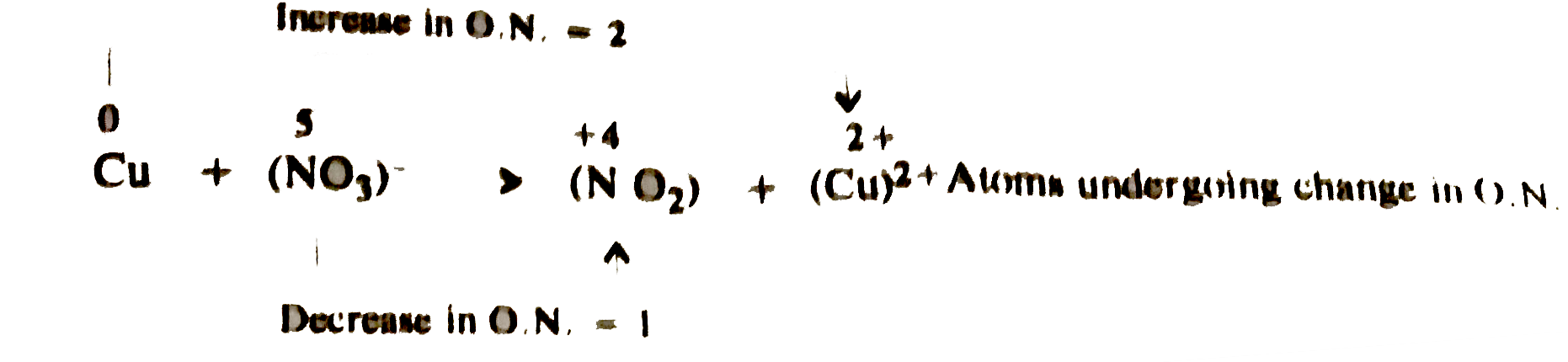Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
DINESH PUBLICATION|Exercise MCQs|42 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Revision Question|92 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|15 VideosSOLID STATE
DINESH PUBLICATION|Exercise Brain storming|10 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-REDOX REACTIONS-Ultimate Preparation
- Balance the following redox reaction : Cu+NO(3)^(-)rarrNO(2)+Cu^(2+)...
Text Solution
|
- When copper is treated with a certain concentration of nitric acid, ni...
Text Solution
|
- For the reaction M^(x+)+MnO(4)^(-)rarrMO(3)^(-)+Mn^(2+)+(1//2)O(2) ...
Text Solution
|
- The number of electrons involved in the reduction of nitrate (NO(3)^(ө...
Text Solution
|
- The oxidation number of S in Na(2)S(4)O(6) is
Text Solution
|
- A compound of Xe and F is found to have 53.5% Xe. What is the oxidatio...
Text Solution
|
- The number of moles of KMnO(4) required to oxidise 1 mol of Fe(C(2)O(4...
Text Solution
|
- In a reaction, 4 mole of electrons are transferred to 1 mole of HNO(3)...
Text Solution
|
- If equal volumes of 1 M KMnO(4) and 1M K(2)Cr(2)O(7) solutions are all...
Text Solution
|
- Equivalent weight of MnO(4)^(-) in acidic, neutral and basic media are...
Text Solution
|