A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE|67 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise MATCH-MATRIX|7 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosD-AND -F BLOCK ELEMENTS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosETHERS
DINESH PUBLICATION|Exercise (MCQs)|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ELECTROCHEMISTRY-REVISION QUESTIONS FROM COMPETITIVE EXAMS
- Aluminium oxide may be electrolysed at 1000^(@)C to furnish aluminium ...
Text Solution
|
- The highest electrical conductivity of the following aqueous solutions...
Text Solution
|
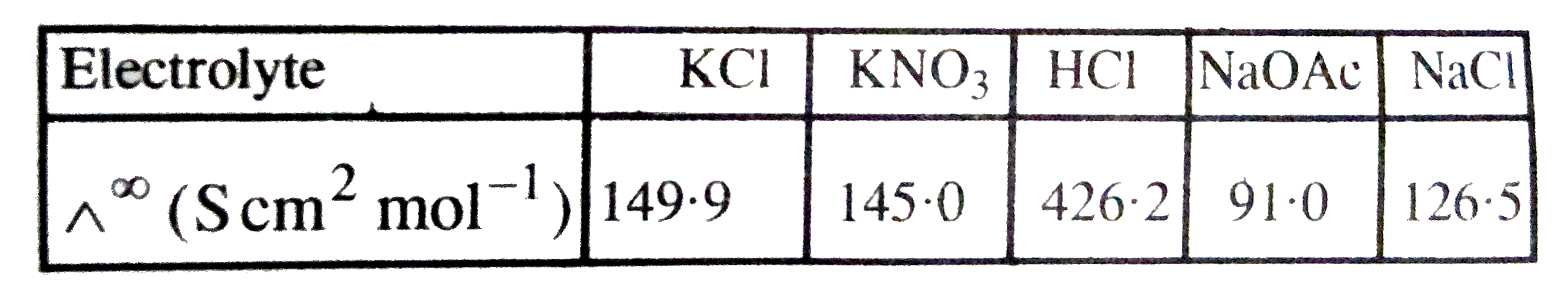
- Calculate ^^overset(oo)HOAc using appropriate molar conductances of th...
Text Solution
|
- How many coulombs are required for the reduction of 1 mol of MnO(4)^(-...
Text Solution
|
- The chemical reaction 2AgCl("(fused)")+H(2(g))rarr2HCl((aq))+2Ag((s)...
Text Solution
|
- The conductivity of 0.001 M acetic acid is 5xx10^(-5)S cm^(-1) and ^^^...
Text Solution
|
- The electrical resistance of a column of 0.04M NaOH solution of diamet...
Text Solution
|
- In electrolysis of dilute H(2)SO(4) what is liberated at anode?
Text Solution
|
- When an electric cell is charged then
Text Solution
|
- The half cell reaction for rusting of iron are: 2H^(+)+2e^(-)+(1)/(2...
Text Solution
|
- For a spontaneous reaction, DeltaG, equilibrium constant (K) and E(cel...
Text Solution
|
- 4.5 g of aluminium (at mass 27 u) is deposited at cathode from Al^(3+)...
Text Solution
|
- The volume of H(2) gas at NTP obtained by passing 4 amperes through ac...
Text Solution
|
- If equivalent conductance of 1 M benzoic acid is 12.8 ohm^(-1) cm^(2) ...
Text Solution
|
- If Zn^(2+)//Zn electrode is diluted 100 times, then the charge in redu...
Text Solution
|
- When a quantity of electricity is passed through CuSO(4) solution 0.16...
Text Solution
|
- Which of the following will increase the voltage of the cell represen...
Text Solution
|
- Which of the following statements are correct concerning redox propert...
Text Solution
|
- The equivalent conductances at infinite dilution of HCl and NaCl are 4...
Text Solution
|
- The molecular conductivity of strong electrolyte
Text Solution
|