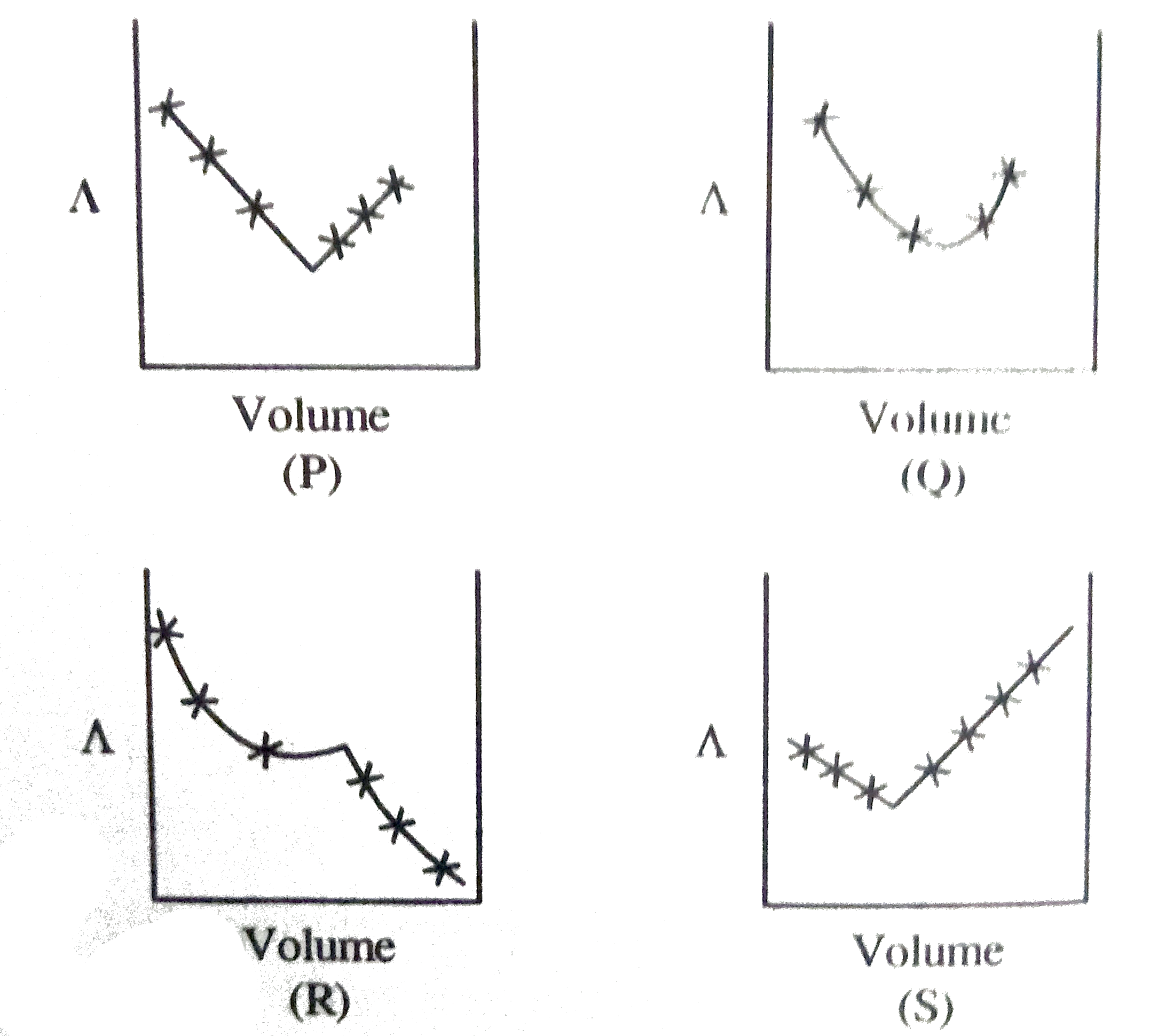
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE|67 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise MATCH-MATRIX|7 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosD-AND -F BLOCK ELEMENTS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosETHERS
DINESH PUBLICATION|Exercise (MCQs)|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ELECTROCHEMISTRY-REVISION QUESTIONS FROM COMPETITIVE EXAMS
- In the electrolysis of which solution OH^(-) ions are discharged in p...
Text Solution
|
- The compound exhibiting maximum conductance in a fused state is
Text Solution
|
- If the half cell reactions are given as (i) Fe^(2+)(aq)+2e^(-)rarrFe...
Text Solution
|
- The logarithm of the equilibrium constant of the cell reaction corresp...
Text Solution
|
- A current is passed through two cells connected in series. The first c...
Text Solution
|
- The limiting molar conductivities of HCl, CH(3)COONa and NaCl are resp...
Text Solution
|
- Standard electrode potential for Sn^(4+)//Sn^(2+) couple is 0.15 V and...
Text Solution
|
- A solution contains Fe^(2+), Fe^(3+) and T^(-) ions. This solution was...
Text Solution
|
- The reduction potential of hydrogen half cell will be negative if :
Text Solution
|
- If E(1),E(2) and E(3) are the emf values of the three galvanic cells ...
Text Solution
|
- Consider the following cell reaction 2Fe(s)+O(2)(g)+4H^(+)(aq)rarr2F...
Text Solution
|
- If the E^(@) for a given reaction has a negative value, then which of ...
Text Solution
|
- Which pair of electrolytes could not be distinguished by the products ...
Text Solution
|
- The electrode potentials for Cu^(2+)+e^(-)toCu^(+) and Cu^(+)e^(-)...
Text Solution
|
- AgNO(3)(aq) was added to an aqueous KCl soltuion gradually and conduct...
Text Solution
|
- Standard reduction potentails of the half reactions are given below: ...
Text Solution
|
- The standard reduction potential for Zn^(2+)//Zn, Ni^(2+)//Ni and Fe^(...
Text Solution
|
- Given E("Cr(2)O(7)^(2-)//Cr^(3+))^(@)=1.33V,E(MnO(4)^(-)//Mn^(2+))^(@)...
Text Solution
|
- A button cell used in watched funcations as follwing Zn(s)+Ag(2)O(s)...
Text Solution
|
- A hydrogen gas electrode is made by dipping platinum wire in a solutio...
Text Solution
|