A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS -Exercise
- The formation of water from H(2)(g) and O(2)(g) is an exothermic proce...
Text Solution
|
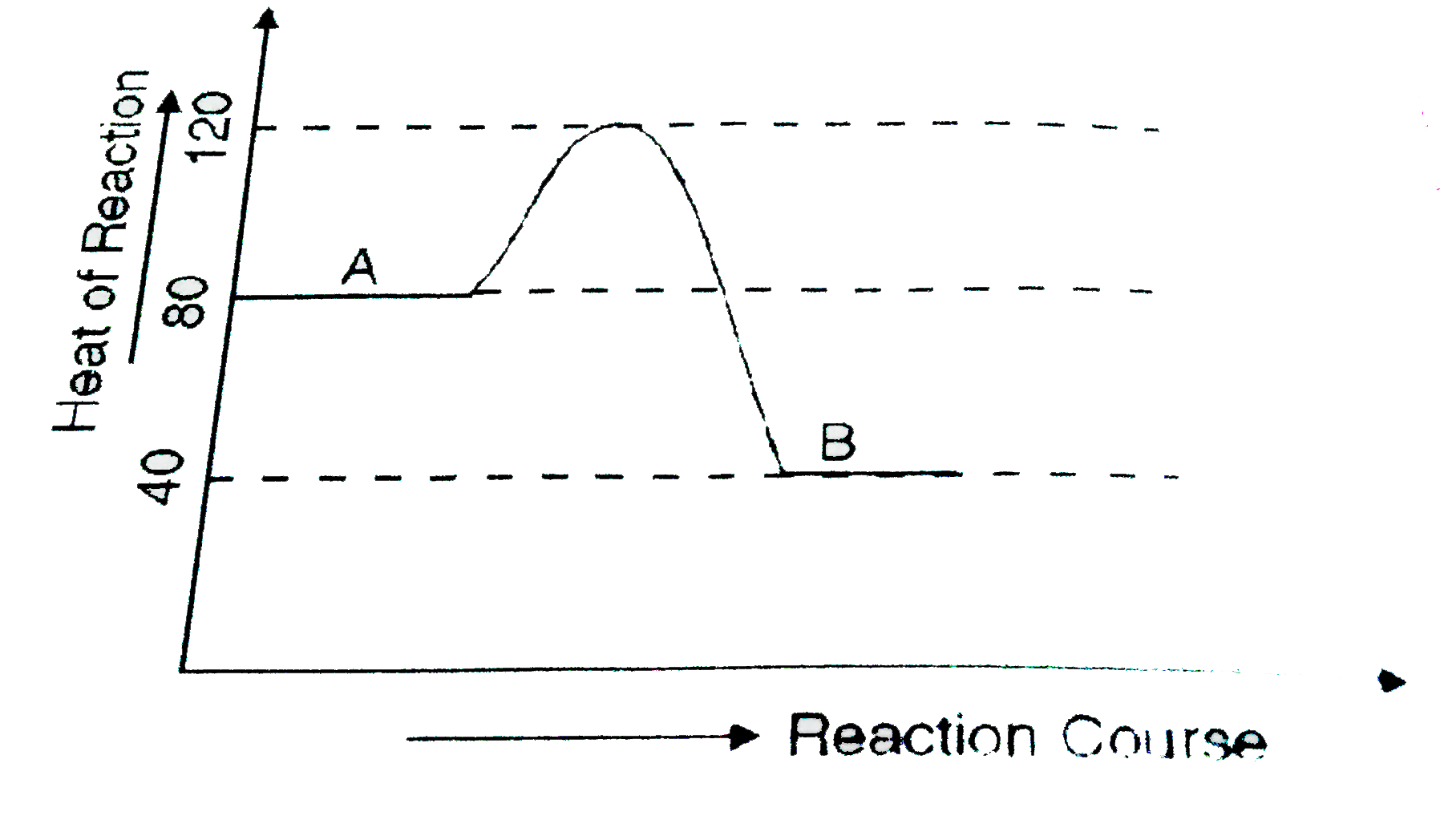
- In a change from state A to state B
Text Solution
|
- According to the diagram given below, the value of DeltaH for conversa...
Text Solution
|
- When ammonium chloride is dissolved in water, the solution becomes col...
Text Solution
|
- The enthalpies of formation of all the elements in their standard stat...
Text Solution
|
- A monoatomic neon molecule possesses
Text Solution
|
- The heat of combusion of benzne determined in a bomb calorimeter is -8...
Text Solution
|
- An endothermic reaction is allowed to take place very rapidly in the a...
Text Solution
|
- The heat absorbed in a reaction at constant temperature and constant v...
Text Solution
|
- In endothermic reactions
Text Solution
|
- For the reaction, 2NH(3)(g)toN(2)(g)+3H(2)(g), which of the followin...
Text Solution
|
- The heat of reaction at constant volume (DeltaE) and that at constant ...
Text Solution
|
- Under which of the following condition is the relation DeltaH = DeltaU...
Text Solution
|
- For the gaseous reaction: N(2)O(4) rarr 2NO(2)
Text Solution
|
- The heat of combustion of solid benzoic acid at constant volume is -32...
Text Solution
|
- For hypothetical reaction A(g) + B(g) to C(g) + D(g) Which of the ...
Text Solution
|
- DeltaE^(@) of combustion of isobutylene is -X kJ mol^(-1). The value o...
Text Solution
|
- The difference between DeltaH and Delta E at constant voluem is equal ...
Text Solution
|
- The reaction given below BaCI(2)(s) +2H(2)O(l) rarr BaCI(2).2H(2)O,D...
Text Solution
|
- Enthalpy change of the reaction 2H(g) to H(2)(g) is -104 kcal The ...
Text Solution
|