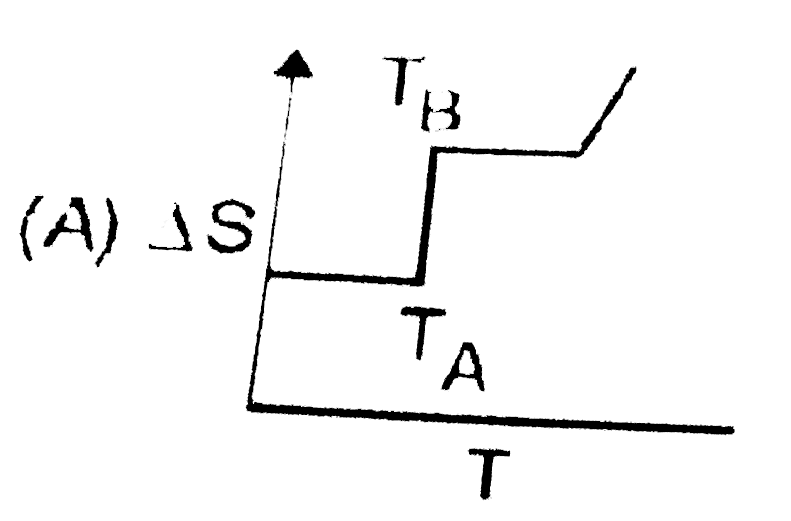
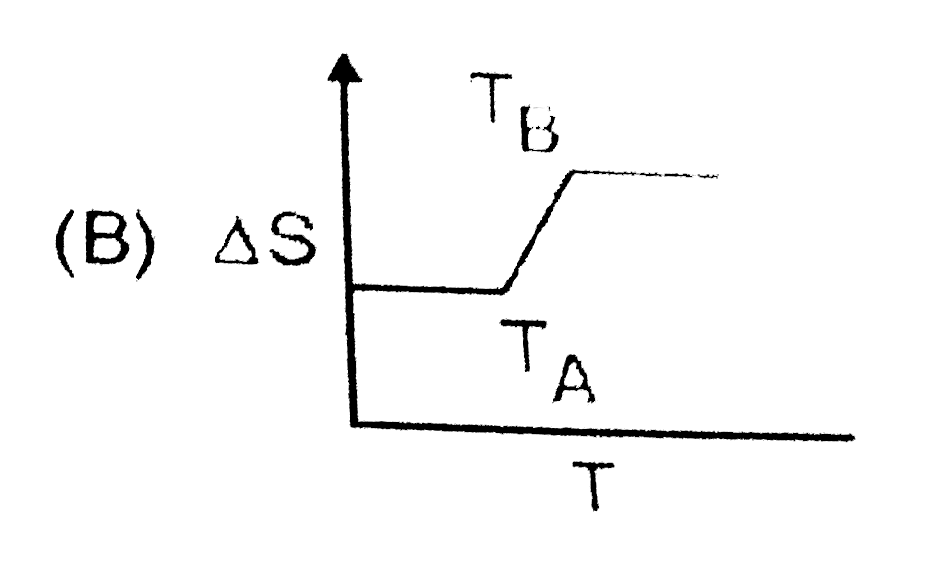
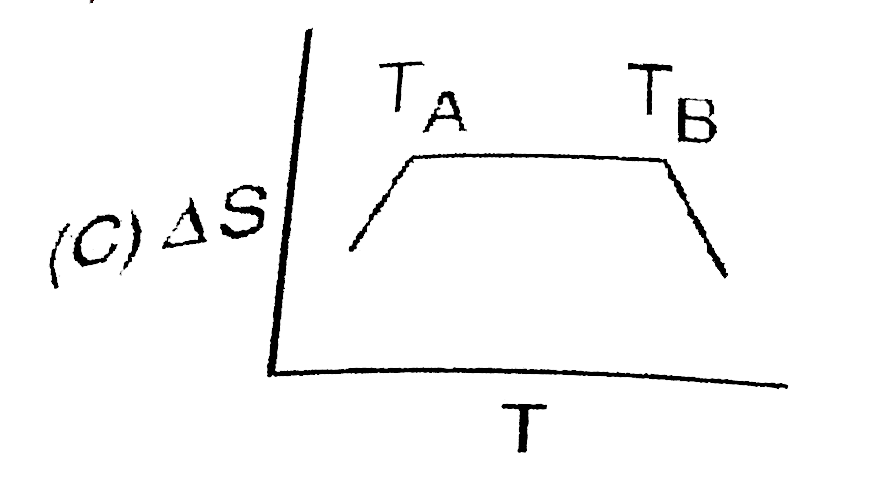
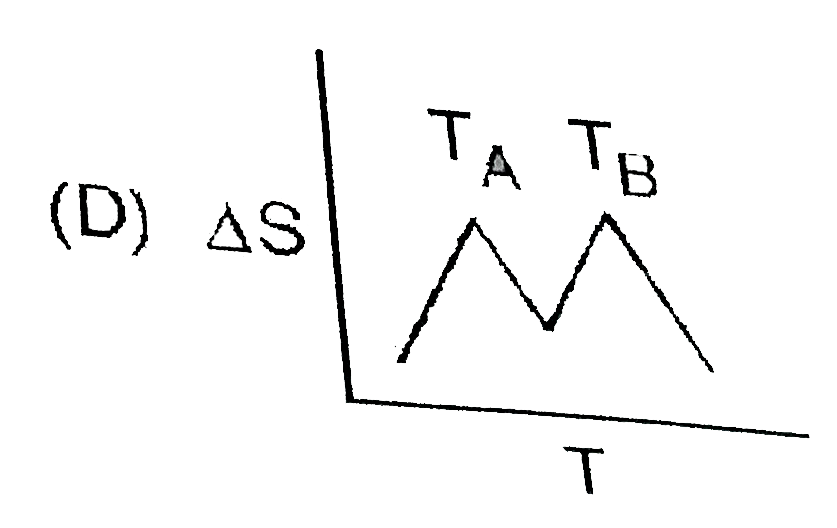A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS -Exercise
- The free energy change for a reversible reaction at equilibrium is
Text Solution
|
- Which of the following conditions regarding the chemical process ensur...
Text Solution
|
- If for a given substance, melting point is T(B) and freezing point is ...
Text Solution
|
- When 1 mole of gas is heated at constant volume. Temperature is raised...
Text Solution
|
- Enthalpy of CH(4)+(1)/(2)O(2)rarrCH(3)OH is negative. If enthalpy of...
Text Solution
|
- The heat required to raise the temperature of a body by 1 K is called
Text Solution
|
- A heat engine absorbs heat Q(1) at temperature T(1) and Q(2) at temper...
Text Solution
|
- An endotthermic reaction is non-spontaneous at freezing point of water...
Text Solution
|
- Compounds with high heat of formation are less stable because
Text Solution
|
- Which of the following statements is true ?
Text Solution
|
- The intensive property among these quantities is
Text Solution
|
- An adiabatic expansion of an Ideal gas always has
Text Solution
|
- The favourable conditions for a spontaneous reaction are
Text Solution
|
- In a closed insulated container, a liquid is stirred with a paddle to ...
Text Solution
|
- C("diamond")toC("Graphite"), DeltaH=-ve. Then shows that
Text Solution
|
- If a reaction involves only solids and liquid, which of the following ...
Text Solution
|
- Mechanical work is specially important in systems that contain
Text Solution
|
- The law formulated by Nernst is
Text Solution
|
- Heat exchanged in a chemical reaction at constant temperature and pres...
Text Solution
|
- Heat of combustion DeltaH^(@) for C(s),H(2)(g) and CH(4)(g) are 94, -6...
Text Solution
|