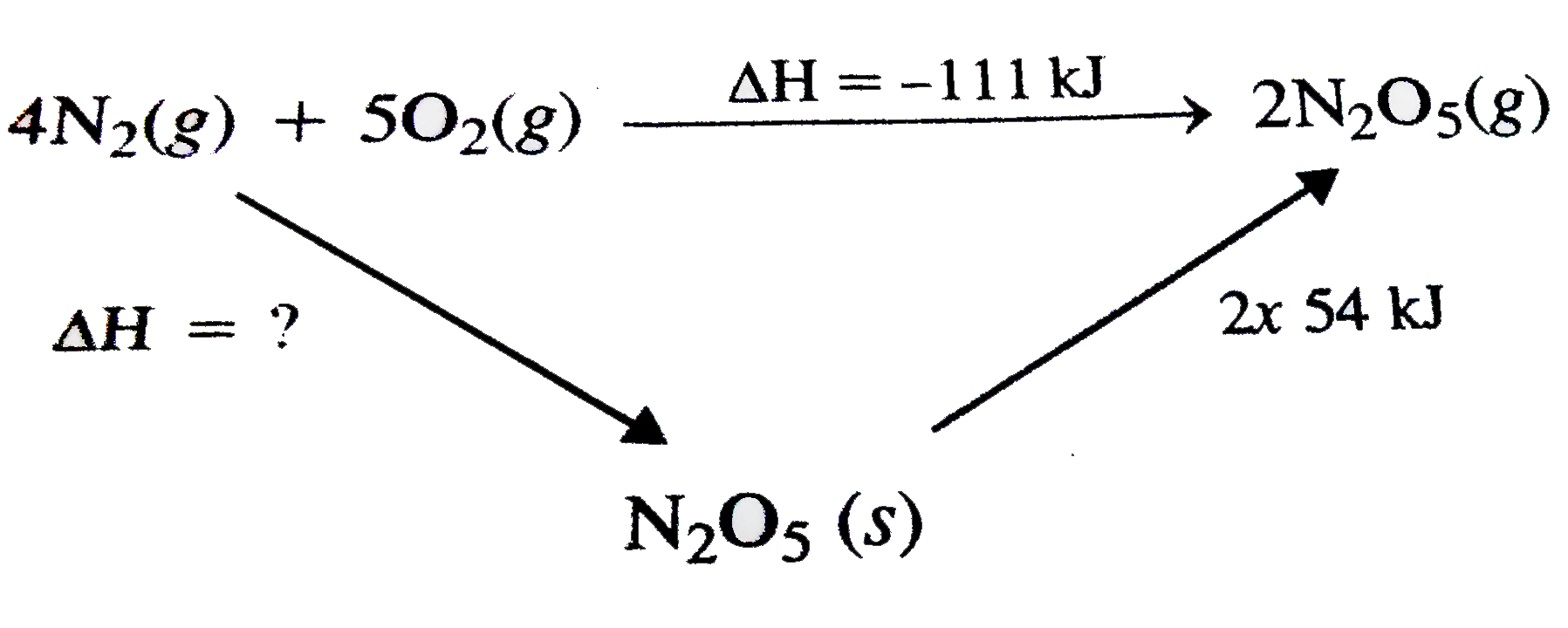
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS -Exercise
- The entropy change involved in the isothermal reversible expansion of ...
Text Solution
|
- Enthalpy change for the reaction 2H(2)(g)rarr4H(g) is -869.6kJ Th...
Text Solution
|
- Consider the reaction, 4NO(2)(g)+O(2)(g)rarr2N(2)O(5)(g),Delta(r )H=...
Text Solution
|
- In view of the signs of Delta(r)G^(0) for the following reactions Pb...
Text Solution
|
- Consider the following processes :- {:(,DeltaH(kJ//mol)),((1)/(2)A r...
Text Solution
|
- At the sublimation temperature, for the process CO(2)(S)toCO(2)(g)
Text Solution
|
- Which of the following sets of conditions would result in a reaction t...
Text Solution
|
- The enthalpy of solution of sodium chloride is 4 kJ mol^(-1) and its e...
Text Solution
|
- The standard enthalpy of formation of C(2)H(4)(l), CO(2)(g) and H(2)O(...
Text Solution
|
- In which of the following reactions,standard reaction entropy change(D...
Text Solution
|
- The enthalpy of fusion of water is 1.435 kcal//"mole". The molar entro...
Text Solution
|
- Standard enthalpy of vaporisationDeltaV(vap).H^(Theta) for water at 10...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- Equal volumes of two monoatomic gases,A,B,at the same temperature and ...
Text Solution
|
- The incorrect expression among the following is
Text Solution
|
- Identify the correct statement from the following in a chemical reacti...
Text Solution
|
- A gasesous system during a thermodynamic process does not undertake an...
Text Solution
|
- Calculate the work done (in joules) when 0.2 mole of an ideal gas at 3...
Text Solution
|
- Which of the following is correct
Text Solution
|
- Choose the reaction with negative DeltaS value
Text Solution
|