A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS -Exercise
- (Delta H - Delta U) for the formation of carbon monoxide (CO) from its...
Text Solution
|
- The enthalpy and entropy change for the reaction, Br(2)(l)+Cl(2)(g)r...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5kJ mol^(-1). If ...
Text Solution
|
- The direct conversion of A to B is difficult, hence is carried out by ...
Text Solution
|
- Consider the following reactions: (i) H^(+)(aq)+OH^(-)(aq)rarrH(2)O(...
Text Solution
|
- Given the bond energies of H - H and Cl - Cl are 430 kJ mol^(-1) and 2...
Text Solution
|
- In conversion of lime-stone to lime, CaCO(3(s)) to CaO((s)) + CO(2(g...
Text Solution
|
- Assuming that water vapour is an ideal gas, the internal energy change...
Text Solution
|
- Identify the correct statement regarding a spontaneous process.
Text Solution
|
- The value of log(10)K for a reaction A hArr B is (Given: Delta(f)H(298...
Text Solution
|
- For the process H(2)O(l) (1 "bar", 373 K) rarr H(2)O(g) (1"bar", 373 K...
Text Solution
|
- The bond dissociation energies for Cl(2), I(2) and IC l are 242.3, 151...
Text Solution
|
- Which of the following are not state functions? (I) q+w (II)q (I...
Text Solution
|
- For the gas phase reaction PCl(5)rarrPCl(3)(g)+Cl(2)(g) which of t...
Text Solution
|
- Bond dissociation enthalpy of H(2) , Cl(2) and HCl are 434, 242 and 43...
Text Solution
|
- Oxidising power of chlorine in aqueous solution can be determined by t...
Text Solution
|
- The bond dissociation energy of B-F in BF(3) is 646 kJ mol^(-1) where...
Text Solution
|
- From the following bond energies H-H bond energy 431.37 kJ mol^(-1) ...
Text Solution
|
- The values of DeltaH and DeltaS for the reaction C("graphite")+CO(2)...
Text Solution
|
- For a particular reversible reaciton at temperature T, DeltaH and Delt...
Text Solution
|
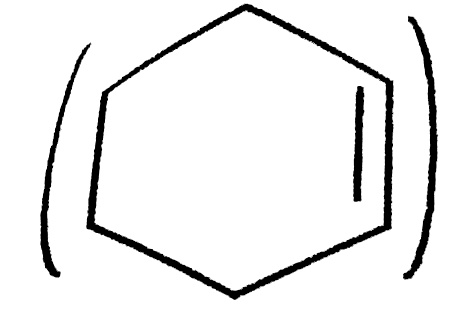 `=-119.5 kJ mol^(-1)`
`=-119.5 kJ mol^(-1)` 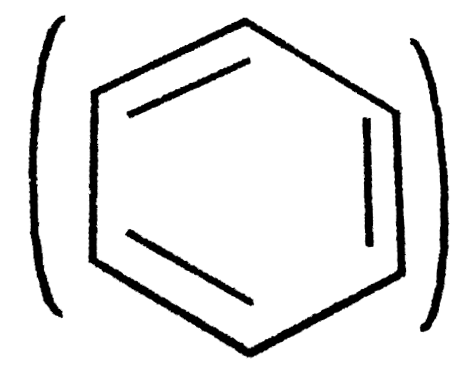 `=3(-119.5)=-358.5 kJ mol^(-1)`
`=3(-119.5)=-358.5 kJ mol^(-1)`