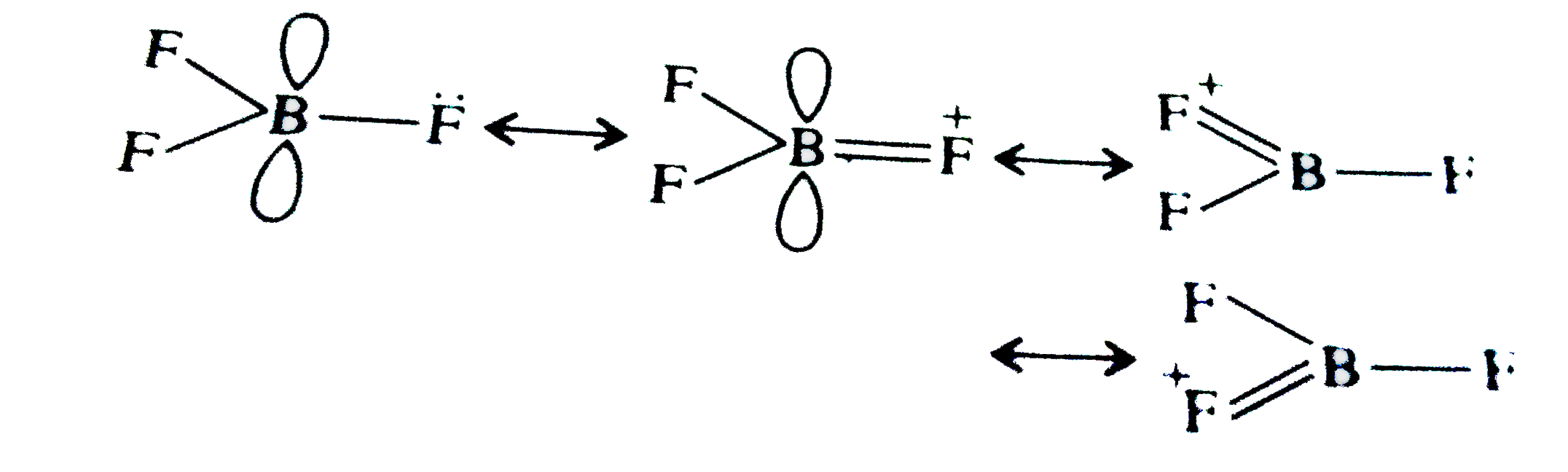
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS -Exercise
- Bond dissociation enthalpy of H(2) , Cl(2) and HCl are 434, 242 and 43...
Text Solution
|
- Oxidising power of chlorine in aqueous solution can be determined by t...
Text Solution
|
- The bond dissociation energy of B-F in BF(3) is 646 kJ mol^(-1) where...
Text Solution
|
- From the following bond energies H-H bond energy 431.37 kJ mol^(-1) ...
Text Solution
|
- The values of DeltaH and DeltaS for the reaction C("graphite")+CO(2)...
Text Solution
|
- For a particular reversible reaciton at temperature T, DeltaH and Delt...
Text Solution
|
- The standard enthalpy of formation of NH(3) is -46.0KJ mol^(-1) . If t...
Text Solution
|
- Standard entropy of X(2) , Y(2) and XY(3) are 60, 40 and 50JK^(-1)mol...
Text Solution
|
- The bond energy (in kcal mol^(-1)) of a C -c single bond is approximat...
Text Solution
|
- The entropy change involved in the isothermal reversible expansion of ...
Text Solution
|
- If the enthaply change for the transition of liquid water to steam is...
Text Solution
|
- Enthalpy change for the reaction 2H(2)(g)rarr4H(g) is -869.6kJ Th...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|