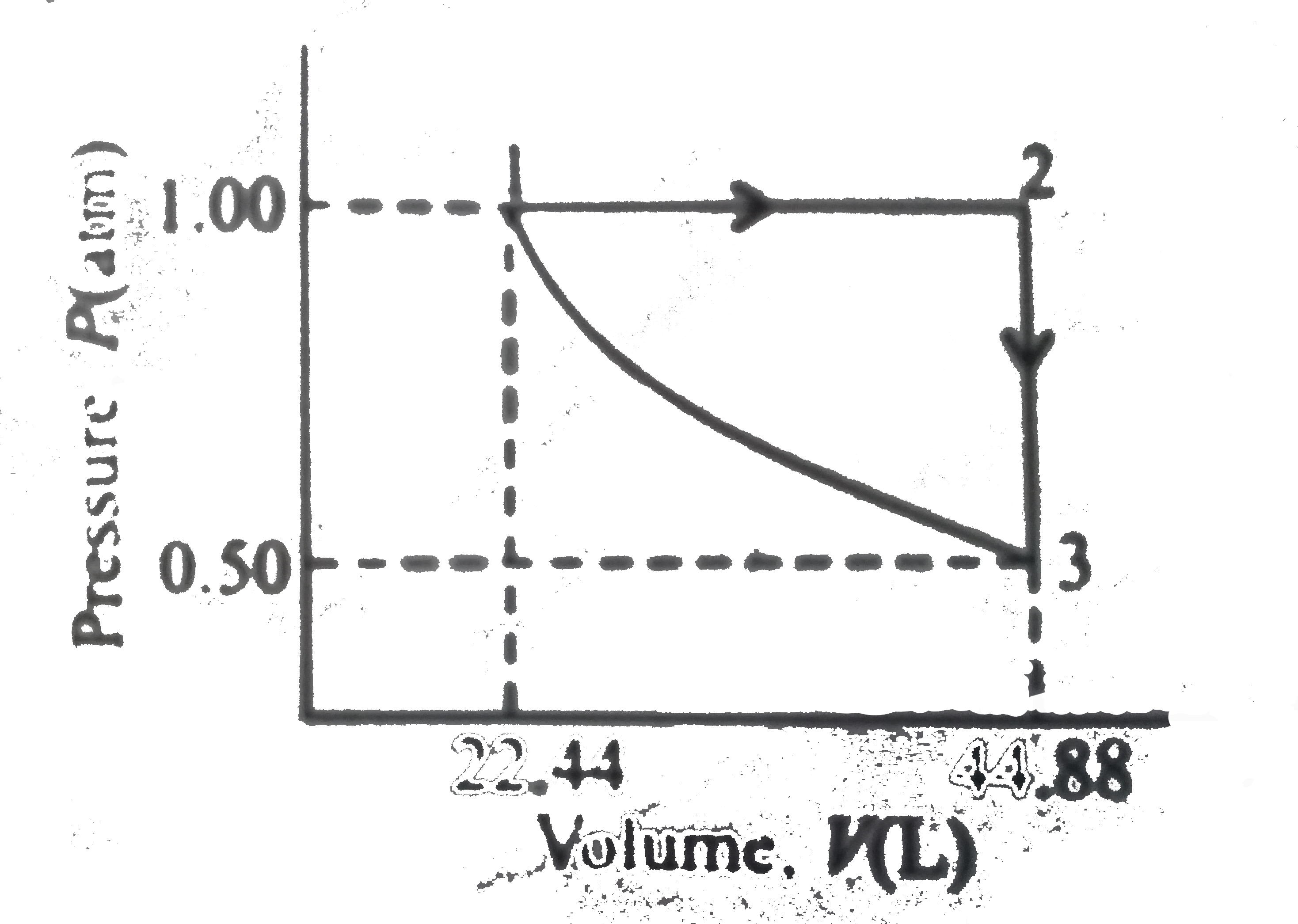
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
DINESH PUBLICATION-CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS -Exercise
- If the enthaply change for the transition of liquid water to steam is...
Text Solution
|
- Enthalpy change for the reaction 2H(2)(g)rarr4H(g) is -869.6kJ Th...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- A gas expands against a constant external pressure so that the work do...
Text Solution
|