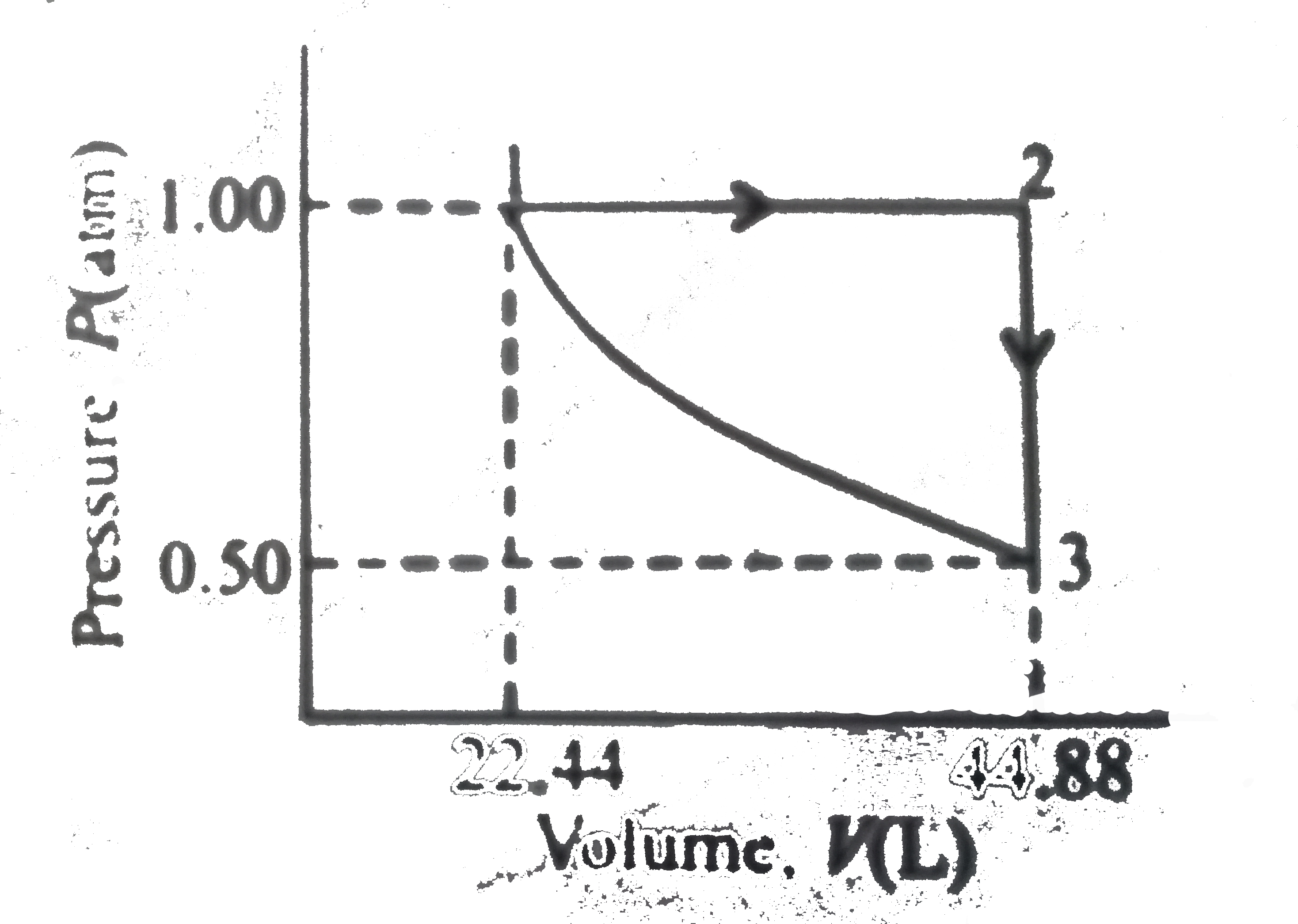
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS -Exercise
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Entropy has great importance in thermodynamics. It is a state function...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- Energy is associated with the orientation and distribution of molecule...
Text Solution
|
- A gas expands against a constant external pressure so that the work do...
Text Solution
|
- The number of state functions the following properties are Temperatu...
Text Solution
|
- In a constant volume calorimeter, 3.5 g of a gas with molecular weight...
Text Solution
|