Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS -Exercise
- The number of state functions the following properties are Temperatu...
Text Solution
|
- In a constant volume calorimeter, 3.5 g of a gas with molecular weight...
Text Solution
|
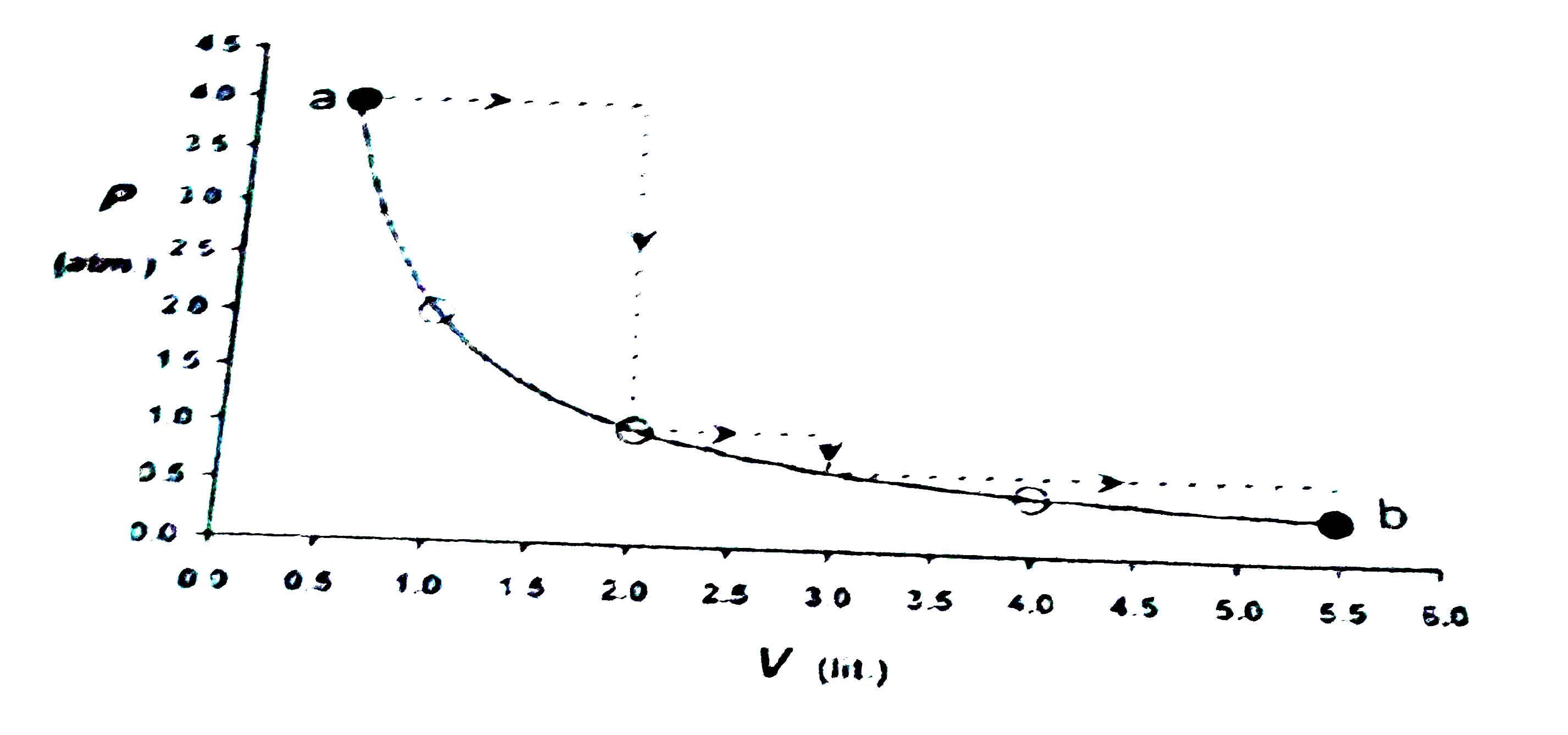
- One mole of an ideal gas is taken from a to b along two paths denoted ...
Text Solution
|
- Assertion(A) : When sodium chloride dissolves in water, then Na^(+) an...
Text Solution
|
- Assertion(A) : The mass and volume of a substance are the extensive pr...
Text Solution
|
- Assertion (A): Enthalpy of graphite is lower than that of diamond. R...
Text Solution
|
- Assertion(A) : For the combustion reactions, the value of DeltaH is al...
Text Solution
|
- Assertion(A) : The value of enthalpy of neutralization of a weak acid ...
Text Solution
|
- Statement-1 : Many endothermic reactions that are not spontaneous at r...
Text Solution
|
- Assertion(A) : Helium has lower entropy than CO(2) gas which has lower...
Text Solution
|
- Assertion: The enthalpy of formation of gaseous oxygen molecules at 29...
Text Solution
|
- Assertion(A) : Heat of vaporisation is always endothermic. Reason(R ...
Text Solution
|
- Assertion(A) : Endothermic compounds are stable than the exothermic co...
Text Solution
|
- Assertion(A) : q is a state function. Reason(R ) : q is a path funct...
Text Solution
|
- Assertion(A) : Heat of neutralisation is always less than zero. Reas...
Text Solution
|
- Assertion(A) : Absolute value of H cannot be determined. Reason(R ) ...
Text Solution
|
- Assertion(A) : Heat of neutralisation of HCl and NaOH is same as that ...
Text Solution
|
- Assertion (A): The dissolution of gases in water is always an endother...
Text Solution
|
- Assertion(A) : The enthalpy of formation of O(2) at 1 atmospheric pres...
Text Solution
|
- Assertion(A) : Two systems which are both in thermal equilibrium with ...
Text Solution
|