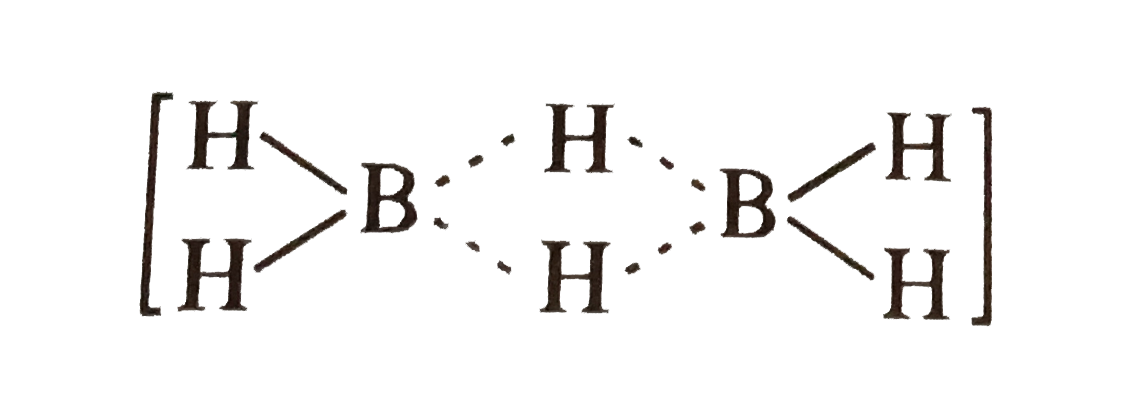A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQs|10 VideosIONIC EQUILIBRIUM
DINESH PUBLICATION|Exercise MCQs with only one correct Answer|29 VideosIONIC EQUILIBRIUM
DINESH PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS|60 VideosHYDROCARBONS
DINESH PUBLICATION|Exercise All Questions|493 VideosNO IDEA
DINESH PUBLICATION|Exercise Unit Test -|1 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-IONIC EQUILIBRIUM -REVISION QUESTIONS FROM COMPETITVE EXAMS
- Which of the following compound will have the smallest pK(a) value ?
Text Solution
|
- The correct order of increasing basicity of the given conjugate bases ...
Text Solution
|
- Which of the following molecular hydrises acts as a Lewis acid?
Text Solution
|
- In aqueous solution the ionization constants for carbonic acid are: ...
Text Solution
|
- The correct order of decreasing acidic nature of H(2)O, ROH, HC -= CH ...
Text Solution
|
- The hydrogen ion concentration of a 10^(-8) M HCl aqueous soultion at ...
Text Solution
|
- 0.023 g of sodium metal is reacted with 100cm^(3) of water. The pH of ...
Text Solution
|
- 0.1M HCl and 0.1 MH(2)SO(4), each of volume 2 ml are mixed and the vo...
Text Solution
|
- The pH of the solutions produced by mixing equal volumes of 2.0 xx 10^...
Text Solution
|
- The solubility product of a sparingly soluble metal hydroxide [M(OH)(2...
Text Solution
|
- Solubility product of silver bromide is 5.0xx10^(-13). The quantity of...
Text Solution
|
- At 25^(@)C, the solubility product of Mg(OH)(2) is 1.0xx10^(-11). At w...
Text Solution
|
- pH of saturated solution of Ba(OH)(2) is 12. The value of solubility p...
Text Solution
|
- What is [H^(+)] in mol//L of a solution that is 0.20 M in CH(3)COONa a...
Text Solution
|
- A weak acid of dissociation constant 10^(-5) is being titrated with aq...
Text Solution
|
- A buffer solution is prepared in which the concentration of NH(3) is 0...
Text Solution
|
- Which of the following is least likely to behave as Lewis acid?
Text Solution
|
- The solubility of Ca(3)(PO(4))(2) in water is y moles // litre. Its so...
Text Solution
|
- pH for the solution of salt undergoing anionic hydrolysis (say CH(3)CO...
Text Solution
|
- The pH of 10^(-2) M Ca(OH)(2) is
Text Solution
|