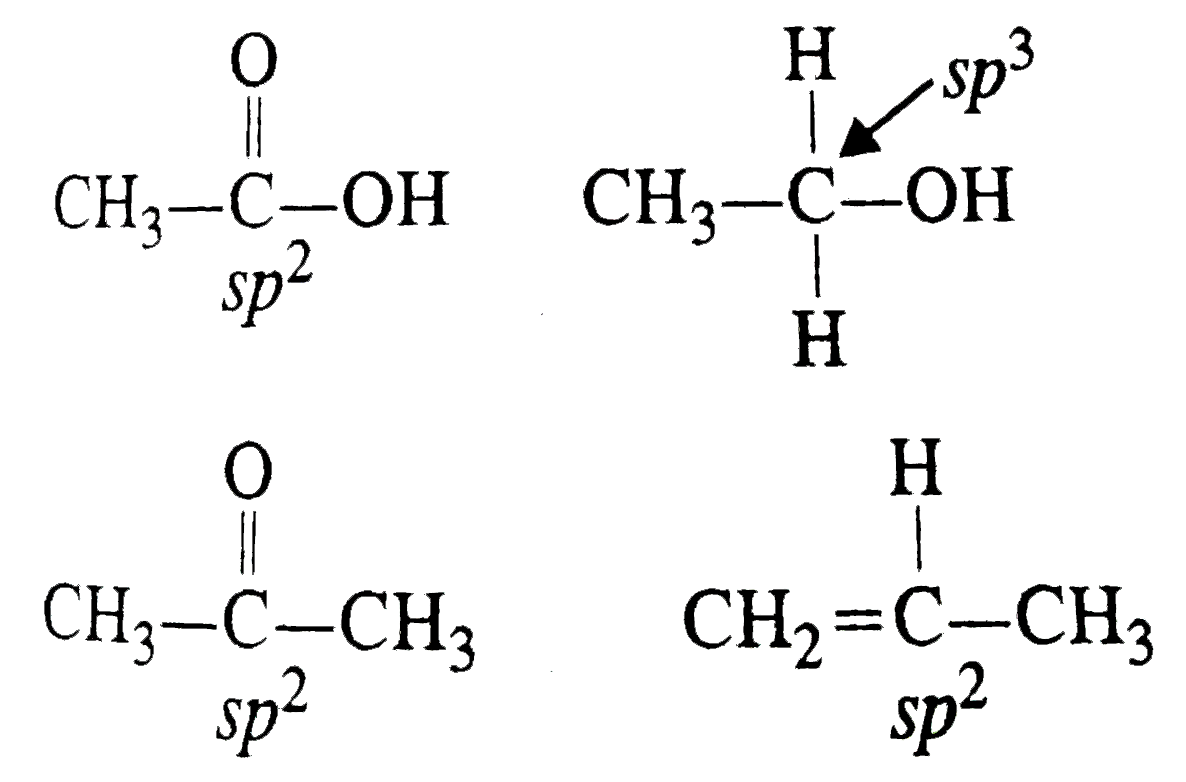A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Comprehension M.C.Q|24 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Matrix Match|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-REVISION
- Which of the following statement is true ?
Text Solution
|
- Number of sigma bonds in P4O10 is
Text Solution
|
- In which of the following sepcies , is the underlined carbon has sp^3 ...
Text Solution
|
- In XeF2, XeF4, and XeF6, the number of lone pairs on Xe is, respective...
Text Solution
|
- The bond order in O2^+ is
Text Solution
|
- Which of the following has zero dipole moment?
Text Solution
|
- The correct sequence of decrease in the bond angles of the following h...
Text Solution
|
- In OF2, the number of bond pairs and lone pairs of electrons are respe...
Text Solution
|
- In NO3^+ ion, number of bond pairs and lone pairs of electrons are res...
Text Solution
|
- In which of the following p pi-d pi bonding is observed ?
Text Solution
|
- Sulphuric acid provides a simple example of
Text Solution
|
- A lone pair of electrons in an atom implies
Text Solution
|
- Chemical bond implies
Text Solution
|
- Sodium chloride is an ionic compound whereas hydrogen chloride is Main...
Text Solution
|
- Covalent compounds have low melting points because
Text Solution
|
- The number of sigma-and pi bonds present in pent-4en-1-yne is :
Text Solution
|
- Which of the following statement is not correct for sigma and pi- bond...
Text Solution
|
- In an anion HCOO^- the two carbon-oxygen bonds are found to be equal l...
Text Solution
|
- The pair of species having identical shapes for molecules of both spec...
Text Solution
|
- The ion which is not tetrahedral in shape is
Text Solution
|