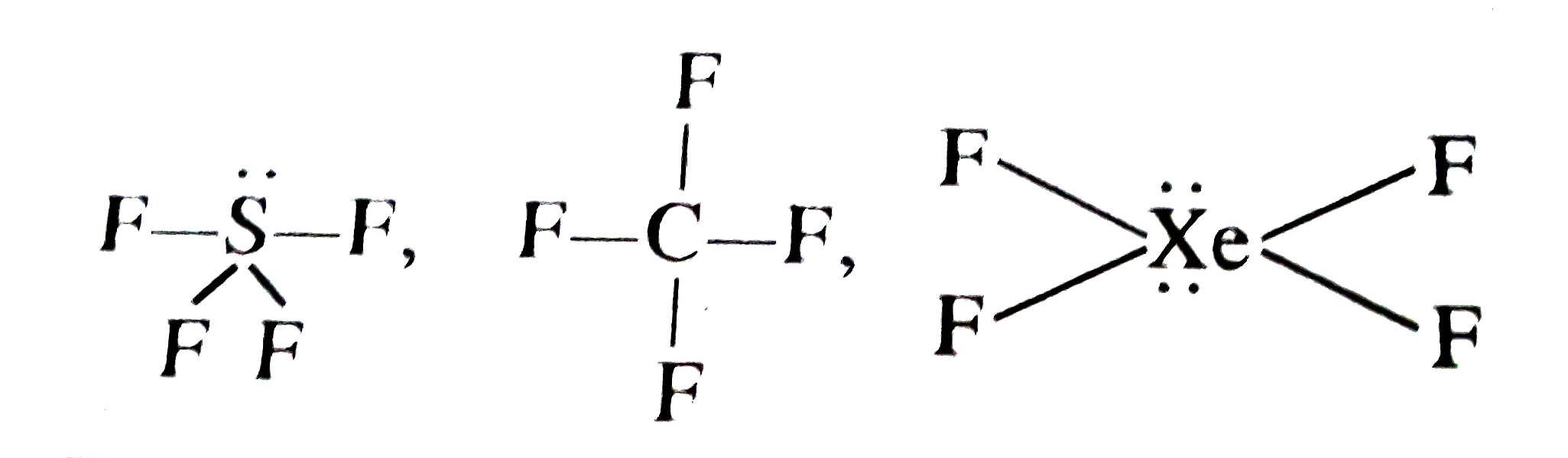A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Comprehension M.C.Q|24 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Matrix Match|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-REVISION
- Identify the correct statement from below concerning the structure of ...
Text Solution
|
- Which carbon is more electronegative ?
Text Solution
|
- Molecular shape of SF4, CF4 and XeF4 are :
Text Solution
|
- The correct order of bond angles (smallest first) in H2 S, NH3, BF3 an...
Text Solution
|
- The states of hybridisation of boron and oxygen atoms in boric acid (H...
Text Solution
|
- Which of the following has the regular tetrahedral structure?
Text Solution
|
- The bond order in NO is 2.5 while that in NO^+ is 3. Which of the foll...
Text Solution
|
- The maximum number of 90^(@) angles between bond pair-bond pair of ele...
Text Solution
|
- Among the species CO2, CO3^(2-), CH3COO^(-), CO, HCHO which has longes...
Text Solution
|
- The boiling point of methanol is greater than methyl thiol because
Text Solution
|
- Two nodal planes are present in
Text Solution
|
- In acetylene molecule, the carbon atoms are linked by:
Text Solution
|
- Shape of O2F2 is similar to that of
Text Solution
|
- The ONO bond angle is maximum in
Text Solution
|
- The dipole moment is the highest for
Text Solution
|
- The decreasing order of bond angle is
Text Solution
|
- H2O is dipolar, whereas BeF2 is not. It is because
Text Solution
|
- In an octahedral structure , the pair of d orbitals involved in d^(2)...
Text Solution
|
- In BrF(3) molecule, the lone pair occupies equatorial position minimiz...
Text Solution
|
- Among the following the pair in which the two species are not isostruc...
Text Solution
|