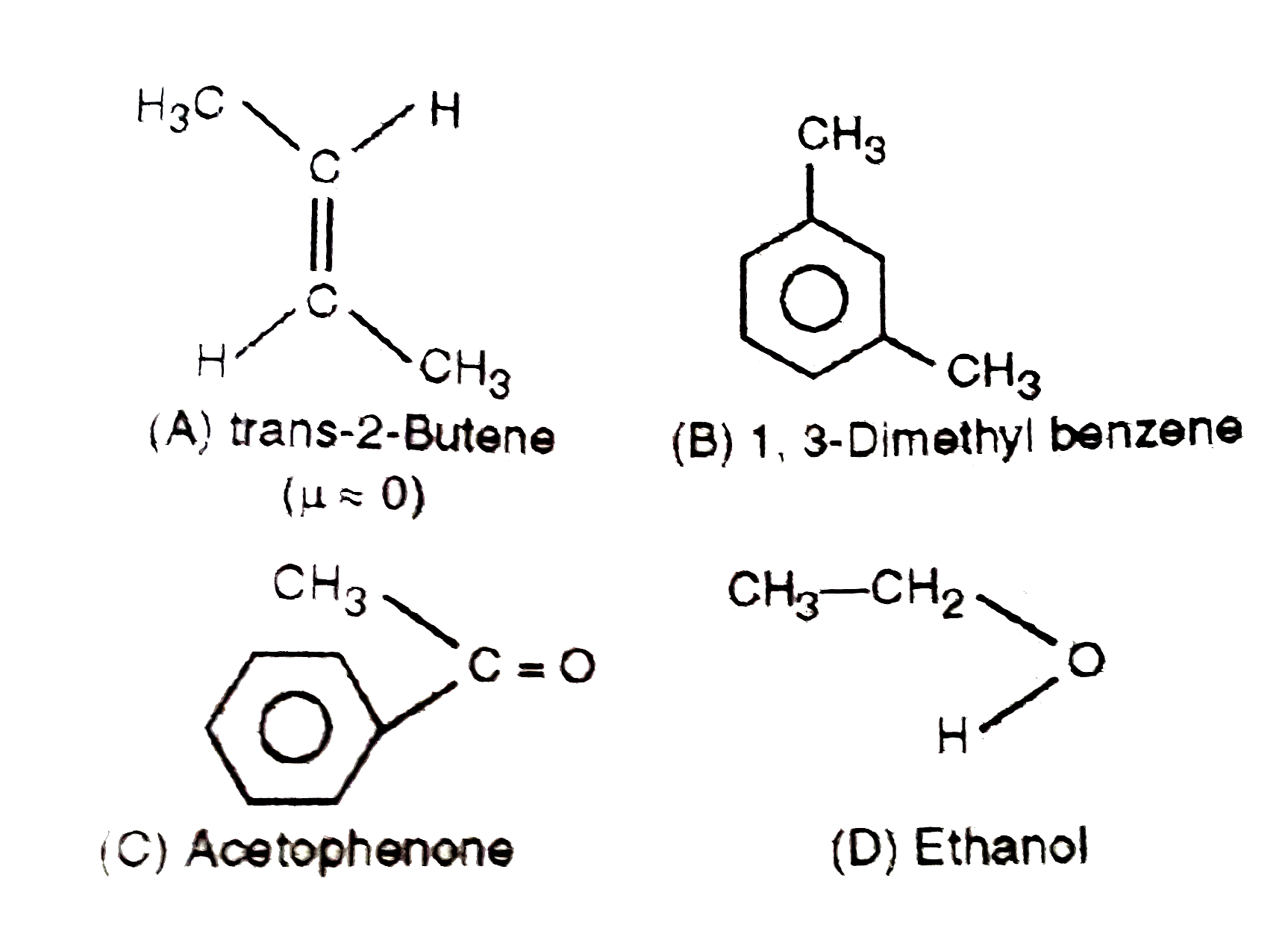
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Comprehension M.C.Q|24 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Matrix Match|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-REVISION
- Shape of O2F2 is similar to that of
Text Solution
|
- The ONO bond angle is maximum in
Text Solution
|
- The dipole moment is the highest for
Text Solution
|
- The decreasing order of bond angle is
Text Solution
|
- H2O is dipolar, whereas BeF2 is not. It is because
Text Solution
|
- In an octahedral structure , the pair of d orbitals involved in d^(2)...
Text Solution
|
- In BrF(3) molecule, the lone pair occupies equatorial position minimiz...
Text Solution
|
- Among the following the pair in which the two species are not isostruc...
Text Solution
|
- Decreasing order of C-C bond length is (I) C2H4 (II) C2H2 (III) C6 H6 ...
Text Solution
|
- How many sigma and pi bonds are present in toluene ?
Text Solution
|
- If the molecule of HCl were totally polar, the expected value of dipol...
Text Solution
|
- Which one of the following molecules has the smallest bond angle ?
Text Solution
|
- Which one of the following sepcies is diamagnetic in nature ?
Text Solution
|
- Lattice energy of an ionic compound depedns upon :
Text Solution
|
- Molecular shape of SF4, CF4 and XeF4 are :
Text Solution
|
- The number and type of bonds between carbon atoms in calcium carbide a...
Text Solution
|
- Of the following sets ,which one does not contain isoeletronic species...
Text Solution
|
- The correct order in which the O-O bond length increases in the follow...
Text Solution
|
- The correct sequence of increasing covalent character is represented b...
Text Solution
|
- Which of the following is electron deficient molecule ?
Text Solution
|