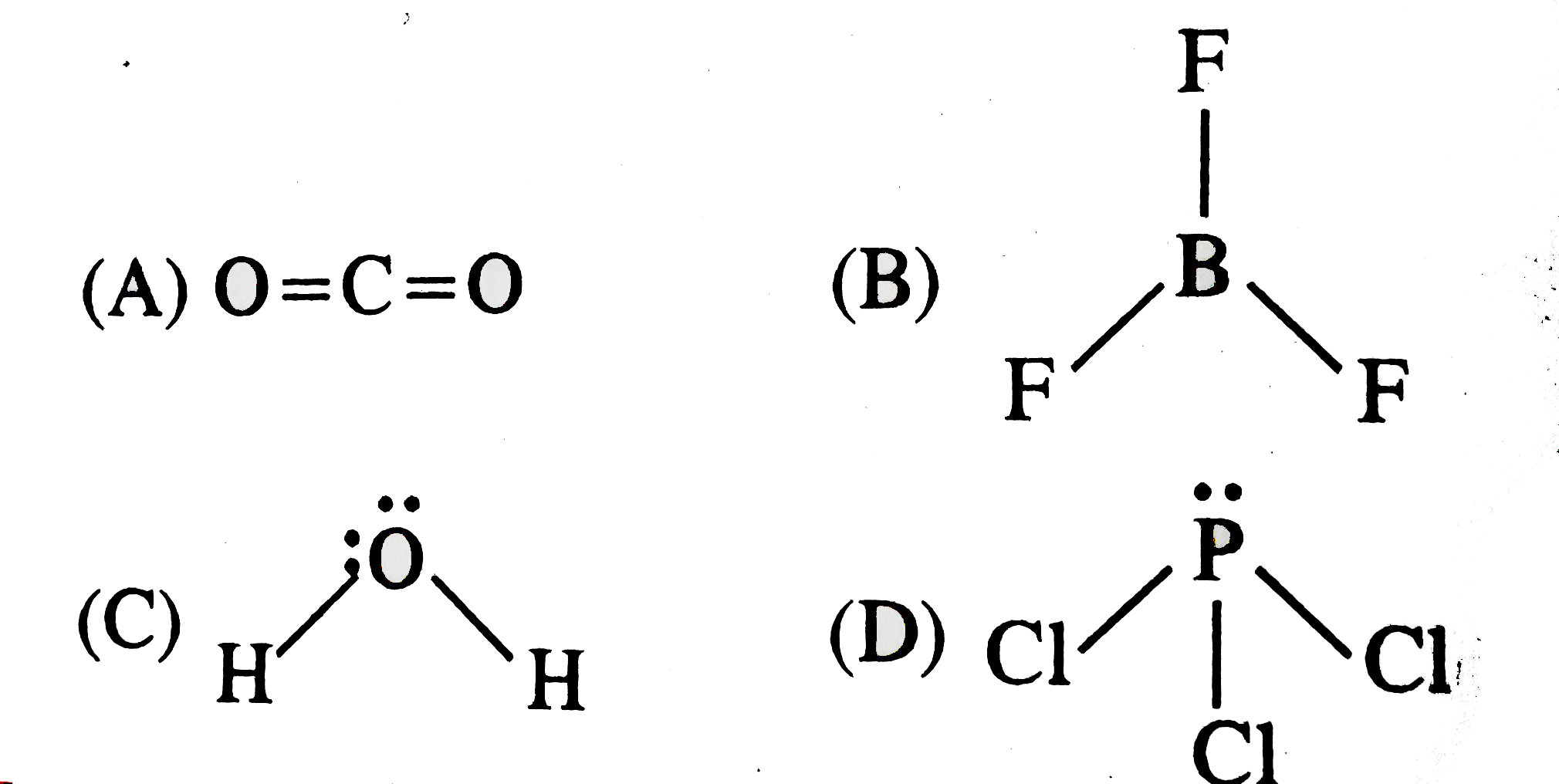
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Comprehension M.C.Q|24 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Matrix Match|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-REVISION
- Which of the following is electron deficient molecule ?
Text Solution
|
- Which of the following would have permanent dipple moment ?
Text Solution
|
- In which of the following molecules the central atom does not follow t...
Text Solution
|
- Which one of the following statements is true ?
Text Solution
|
- The correct order of the lattice energies of the following ionic compo...
Text Solution
|
- The H-O-H bond angle in water is
Text Solution
|
- Which of the following molecule is linear?
Text Solution
|
- Which of the following is correct ?
Text Solution
|
- The hydrogen bond is strongest in
Text Solution
|
- Match list and list II and pick out correct matching from the given ch...
Text Solution
|
- The sequence that correctly describes the relative bond strengths pert...
Text Solution
|
- Consider the following molecules or ions CH2Cl2 (ii) NH4^+ (iii) SO...
Text Solution
|
- The decreasing order of the boiling points of the following hydrides ...
Text Solution
|
- Match list and list II and pick out correct matching from the given ch...
Text Solution
|
- The energy of hydrogen bond is of the order of
Text Solution
|
- The number of lone pairs of electrons present on the central atom of C...
Text Solution
|
- Bond order of nitric oxide is
Text Solution
|
- How many types of F-S-F bonds are present in SF4?
Text Solution
|
- The number of sigma and pi- bonds in allyl isocyanide are
Text Solution
|
- In TeCl4, the central tellurium involves the hybridization
Text Solution
|