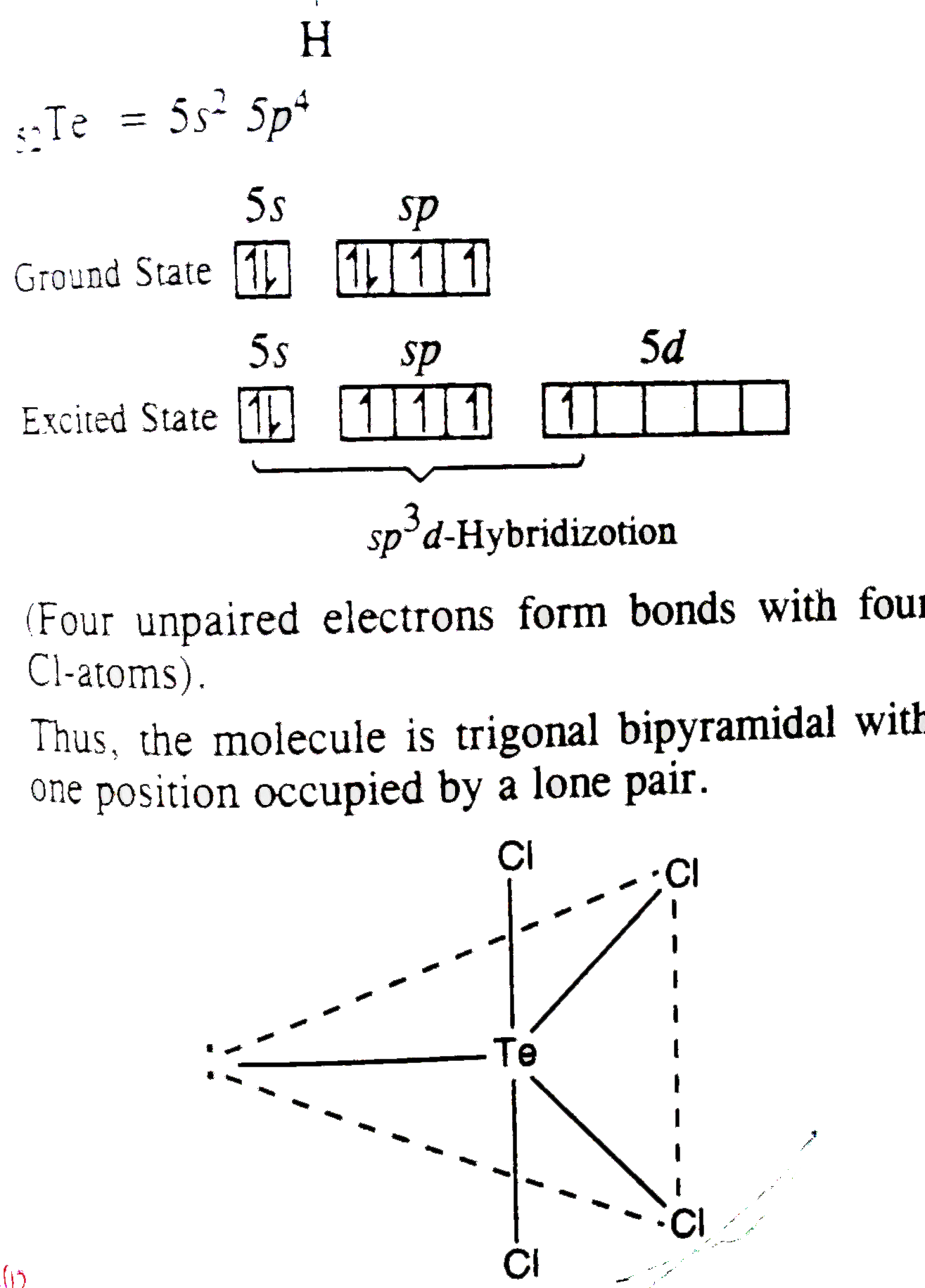A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Comprehension M.C.Q|24 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Matrix Match|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-REVISION
- How many types of F-S-F bonds are present in SF4?
Text Solution
|
- The number of sigma and pi- bonds in allyl isocyanide are
Text Solution
|
- In TeCl4, the central tellurium involves the hybridization
Text Solution
|
- Match the compounds in the List with that in List II {:("List I","Li...
Text Solution
|
- The correct order of bond order values among the following (i) NO^(...
Text Solution
|
- The bond lengths and bond angles in the molecules of methane, ammonia,...
Text Solution
|
- Which one of the following pairs consists of only paramagnetic species
Text Solution
|
- The hybridization of oxygen atom in H2O2 is
Text Solution
|
- Which of the following is paramagnetic with bond order 0.5?
Text Solution
|
- Which of the following is correctly based on molecular orbital theory ...
Text Solution
|
- Which has the highest dipole moment?
Text Solution
|
- The magnetic moment of KO(2) at room temperature is ---------- BM.
Text Solution
|
- Shape of ClF3 is
Text Solution
|
- Which of the following is the structure of N2O which is isoelectronic ...
Text Solution
|
- Which of the following has transient existence?
Text Solution
|
- The number of nodal planes present in a sigma^** antibonding orbital i...
Text Solution
|
- The calculated bond order of superoxide ion (O2^(-)) is
Text Solution
|
- The bond angle and dipole moment of water respectively are :
Text Solution
|
- Match list (Molecules) with list II (Boiling points) and select the co...
Text Solution
|
- Shape and hybridization of IF5, respectively, are
Text Solution
|