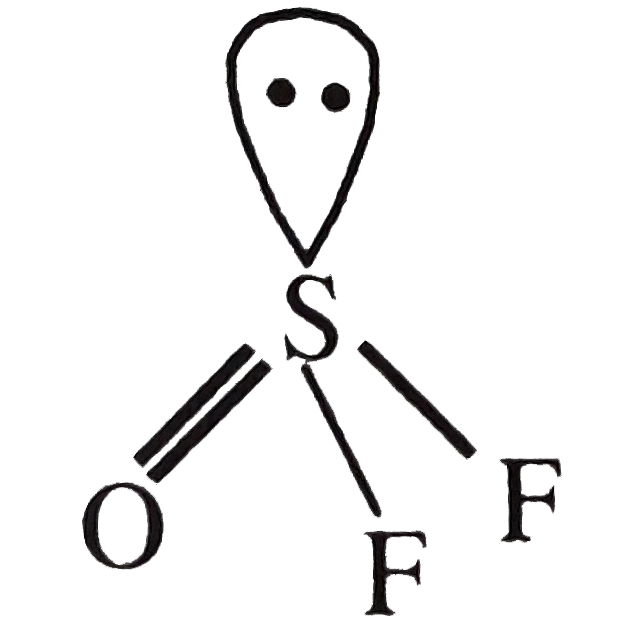
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Comprehension M.C.Q|24 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Matrix Match|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-REVISION
- How many sigma(sigma) bonds are there in CH2=CH-CH = CH2 ?
Text Solution
|
- How many sigma and pi bonds are present in the given compound Ph-CH=un...
Text Solution
|
- The species having pyramidal shape is
Text Solution
|
- Peroxide ion…… (i) has five completely filled antibonding molec...
Text Solution
|
- In which of the following molecules, the central atom does not have sp...
Text Solution
|
- Some of the properties of the two species NO(2)^(-) and H(3)O^(+) are ...
Text Solution
|
- Find the bond order of CO
Text Solution
|
- Which of the following has a regular geometry
Text Solution
|
- A neutral molecule XF has a zero dipole moment. The X is most likely
Text Solution
|
- N2 and O2 are converted into mono positive cations N2^+ and O2^+ respe...
Text Solution
|
- Which of the following statement is false
Text Solution
|
- The bond angle formed by different hybrid orbitals are in the order
Text Solution
|
- Assuming that Hund's rule is violated the bond order and magnetic natu...
Text Solution
|
- In which of the following molecules is hydrogen bridge bond present ?
Text Solution
|
- Complete the following reaction
Text Solution
|
- In which one of the following species , the central atom has the tuype...
Text Solution
|
- Which of the following conversion involves change in both hybridizatio...
Text Solution
|
- The correct order of increasing bond angle in the following species is
Text Solution
|
- Which of the following is a the most preferred and hence of the lower ...
Text Solution
|
- In which of the following, the central atoms has two lone pairs of ele...
Text Solution
|