Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Reason Assertion|37 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|20 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Matrix Match|4 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Integer
- The number of unpaired electrons in O2 is ……
Text Solution
|
- In Al2Cl6 each Al atoms is linked to how many Cl atoms ?
Text Solution
|
- The number of water molecule(s) derectly bonded to the metal centre in...
Text Solution
|
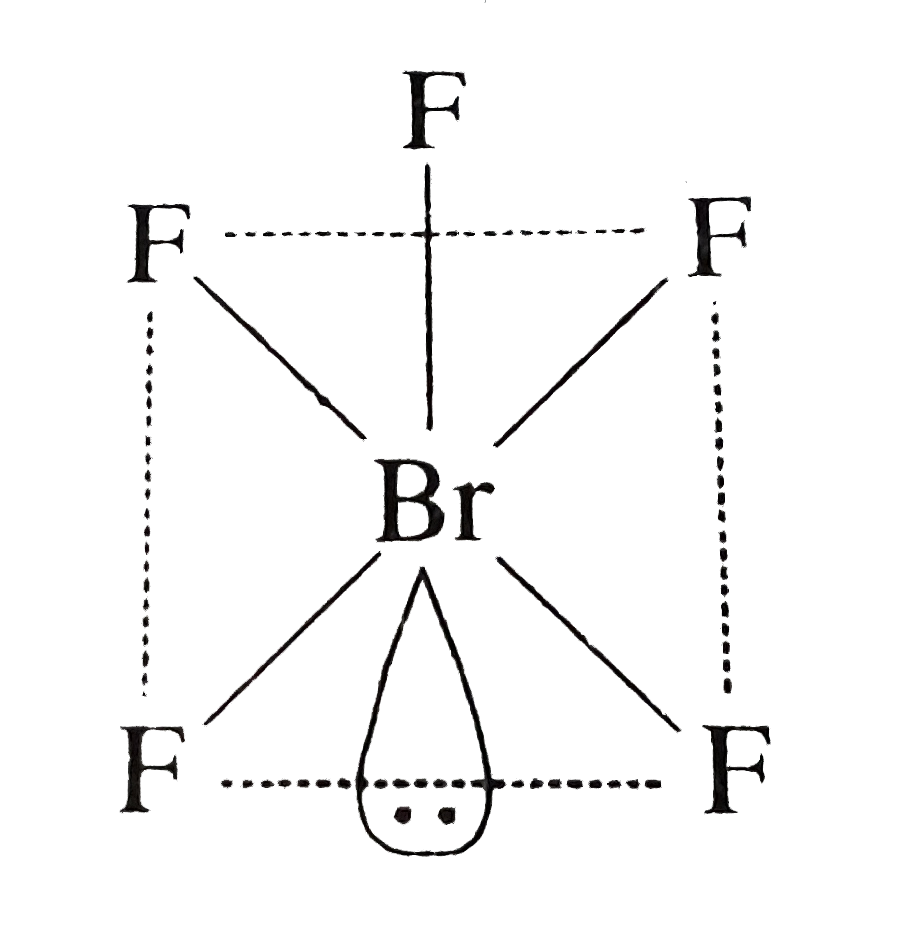
- Based on VSEPR theory, the number of 90 degree F-B-F angles in BrF5 is...
Text Solution
|