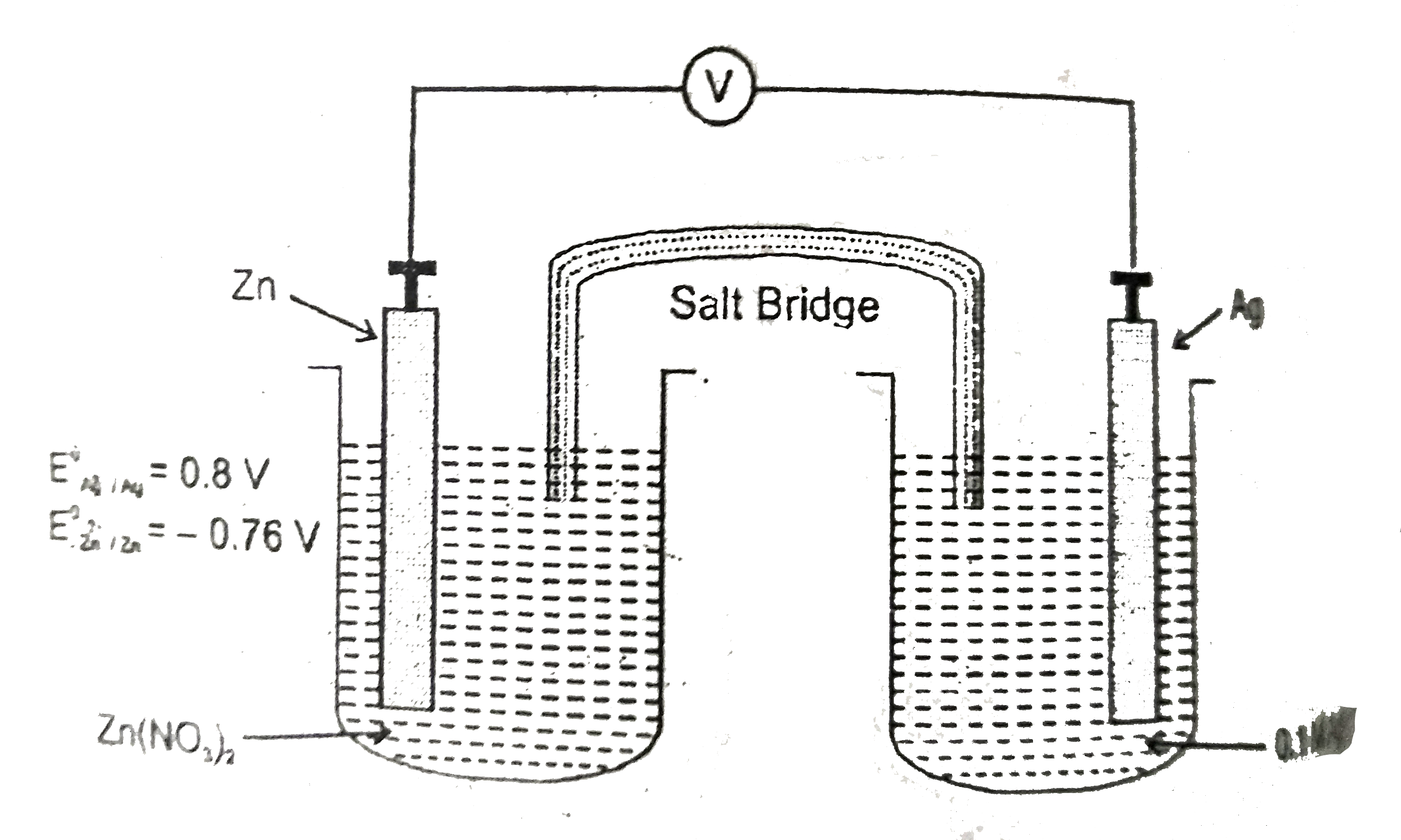
Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
DINESH PUBLICATION|Exercise PROBLEMS FOR PRACTIVE|56 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise QUESTIONS FROM BOARD EXAMINATIONS|81 VideosD-AND -F BLOCK ELEMENTS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosETHERS
DINESH PUBLICATION|Exercise (MCQs)|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ELECTROCHEMISTRY-HOTS
- The curves obtained when molar conductivity lambda(m) (along Y-axis) i...
Text Solution
|
- The figure shows two electrolytic cells connected in series (a) How ...
Text Solution
|
- In the dry cell (a) Which substance acts as anode and which as catho...
Text Solution
|
- Two platinum electrides are dipped in an aqueous solution of copper su...
Text Solution
|
- For the redox reaction : Zn(s)+Cu^(2+)(aq) hArr Zn^(2+)(aq)+Cu(s) ...
Text Solution
|
- Consider the following electrochemical cell. (a). Write a balanced n...
Text Solution
|
- Magnesium metal is produced commercially by the isolation of MgCl(2) f...
Text Solution
|
- (a) In a cell reaction, equilibrium constant L is less than one. Is E^...
Text Solution
|
- A silver oxide-zinc cell maintains a fairly constant voltage during di...
Text Solution
|
- A constant current of 30 amperes ispassed through an aqueous solution ...
Text Solution
|
- Calculate the value of equilibrium constant for the reaction taking pl...
Text Solution
|
- The K(sp) for AgCl at 298 K is 1.0xx10^(-10). Calculate E for Ag^(+)//...
Text Solution
|
- The reduction potentials of Cu^(2+)//Cu and Ag^(+)//Ag electrodes are ...
Text Solution
|
- The standard reduction potential for the half-cell, NO(3(aq.))^(-)+2H(...
Text Solution
|
- The standard reduction potential of the Ag^(+)//Ag electrode at 298 K ...
Text Solution
|
- How many grams of silver could be plated out on a serving tray be elec...
Text Solution
|
- A 100 W and 110 V incandescent lamp is connected in series with an ele...
Text Solution
|