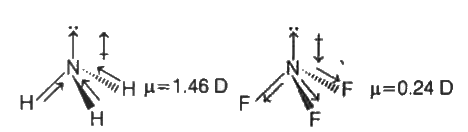A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-ONLINE QUESTION PAPER -CHEMISTRY
- Which of the following are correct ? (1) Electron density in XY plane...
Text Solution
|
- What is the correct order of atomic/ionic size ?
Text Solution
|
- Identify the correct statements from the following. (1) The dipole m...
Text Solution
|
- Identify the pair that is not isostructural
Text Solution
|
- Find the odd-electron molecules from the following. underset((i))(C(...
Text Solution
|
- The ratio between the RMS velocity of N(2) at 200K and that of CO at 8...
Text Solution
|
- For a fixed mass of an ideal gas the correct representation is
Text Solution
|
- The amount of iron (Fe) in g which can be produced from 600g of magnet...
Text Solution
|
- If stoichiometric quantities of KMnO(4) and K(2)Cr(2)O(7) mixture is a...
Text Solution
|
- A sample of argon of 1 atm pressure and 300K expands reversibly and ad...
Text Solution
|
- If equilibrium constant of a process is 3.8 xx 10^(-3) " at" 25^(@)C, ...
Text Solution
|
- Which of the following compound give basic solution on hydrolysis ? ...
Text Solution
|
- Hardness of water is 200 ppm. Calculate the molarity and normality of ...
Text Solution
|
- Which pair of elements on combustion in air give superoxides ?
Text Solution
|
- When borax is dissolved in water, the product formed is
Text Solution
|
- SiO(2) reacts with
Text Solution
|
- Pure water would have a BOD value of
Text Solution
|
- Tropolone is
Text Solution
|
- Newman projection of staggered conformation of ethane is
Text Solution
|
- 2-pentyne on reaction with sodium in liquid ammonia produced compound ...
Text Solution
|