A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION B : Objective Type Questions)|20 VideosCHEMICAL KINETICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION C : Previous Year Questions)|62 VideosCHEMICAL KINETICS
AAKASH INSTITUTE|Exercise EXERCISE|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section J (Aakash Challengers Questions)|10 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH INSTITUTE|Exercise Assignment ( SECTION - A)|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-CHEMICAL KINETICS-ASSIGNMENT (SECTION A : Objective Type Questions)
- For a lst order reaction , a straight line is obtained if you plot
Text Solution
|
- Which order reaction obeys the expression t(1//2)prop1[A] ?
Text Solution
|
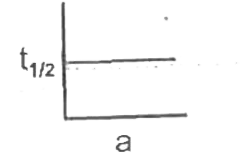
- The graph of t(1//2) versus initial concentration 'a' is for
Text Solution
|
- Reactant 'A' (initial concentration, a) reacts according to zero order...
Text Solution
|
- A first order reaction completes 60% 20 minutes. The time required for...
Text Solution
|
- The half life period of a substance is 50 minutes at a certain initial...
Text Solution
|
- The half life of a second order process, 2A rarr products , is
Text Solution
|
- The rate law for the reaction RCl + NaOH(aq) rarr ROH + NaCl is give...
Text Solution
|
- The rate constant for forward and backward reactions of hydrolysis of ...
Text Solution
|
- For a reaction , A rarrB , it has been found that the order of the re...
Text Solution
|
- A sample of a radioactive substance undergoes 80% decomposition in 345...
Text Solution
|
- 99% at a first order reaction was completed in 32 min. When will 99.9%...
Text Solution
|
- Check, which of the following statements is false ?
Text Solution
|
- Arrhenius parameter (A) depends on
Text Solution
|
- The chemical reactions in which the reactants require high amount of a...
Text Solution
|
- For an exothermic chemical process ocuuring in two process occuring in...
Text Solution
|
- A chemical process occurring in two steps , is plotted as
Text Solution
|
- For the chemical process energies are plotted in graph. Which of ...
Text Solution
|
- The rate of a reaction becomes 2 times for every 10^@C rise in tempera...
Text Solution
|
- The rate of a reaction increases by 2.5 times when the temperature is ...
Text Solution
|