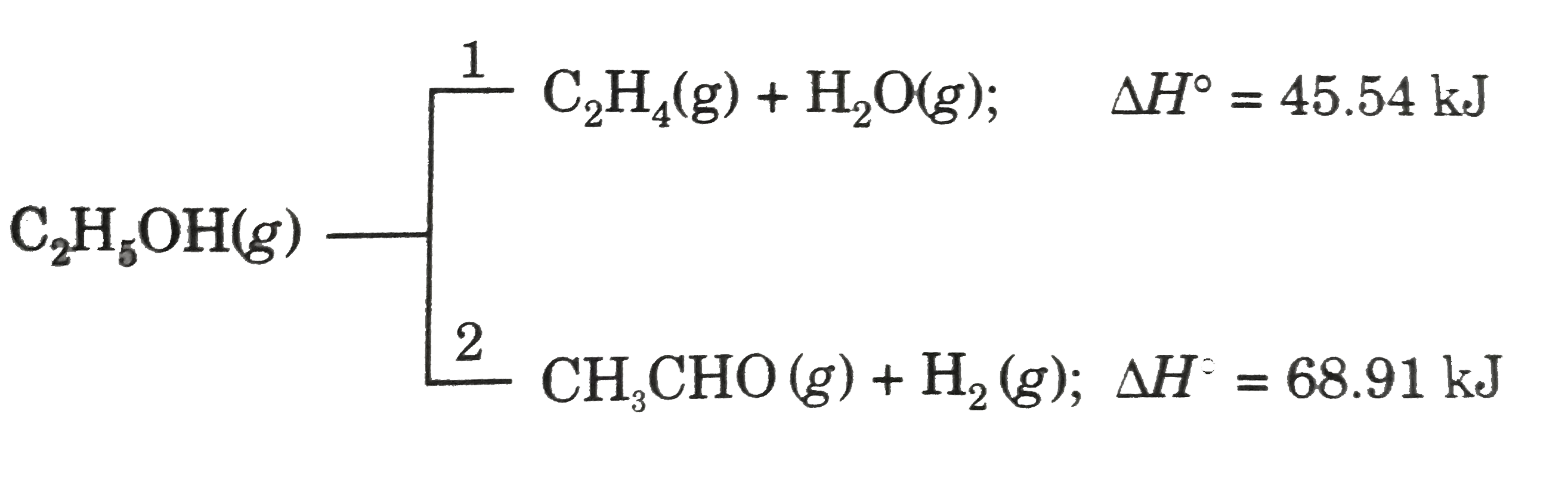A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-THERMOCHEMISTRY-Exercise 1
- For which change Delta H ne Delta E :-
Text Solution
|
- Reactions involving gold have been of particular interest to a chemist...
Text Solution
|
- If x(1),x(2) and x(3) are enthalpies of H-H , O=O and O-H bonds respec...
Text Solution
|
- For the allotropic change represented by the equation C (graphit) rarr...
Text Solution
|
- Given : (i) NH(3) (g) + 3Cl(2)(g) rarr NCl(3)(g)+3HCl(g), Delta H(1)...
Text Solution
|
- Ethanol can undergo decompostion to form two sets of products. If th...
Text Solution
|
- Find Delta(r)U^(@) for the reaction 4HCl (g) + O(2) (g) rArr 2Cl(2) (g...
Text Solution
|
- The enthalpy change of the following reactions at 27^(@)C are {:(Na...
Text Solution
|
- What is the ratop of the enthalpy yield on combustion of hydrogen of h...
Text Solution
|
- The molar heat capacities at constant pressure (assume constant with r...
Text Solution
|
- The lattice enthalpy of solid NaCl is 772 kJ mol^(-1) and enthalpy of ...
Text Solution
|
- DeltaH(f)^(@) of water is -285.5 KJ "mol"^(-1). If enthalpy of neutral...
Text Solution
|
- Select the correct option.
Text Solution
|
- The standard enthalpy of formation of ammonia gas is Given: N(2)H(4...
Text Solution
|
- Study the following therochemical equations: {:(A,rarr,B,,,Delta H ...
Text Solution
|
- Select the option in which heat evolved is maximum. Given : Delta(f)...
Text Solution
|