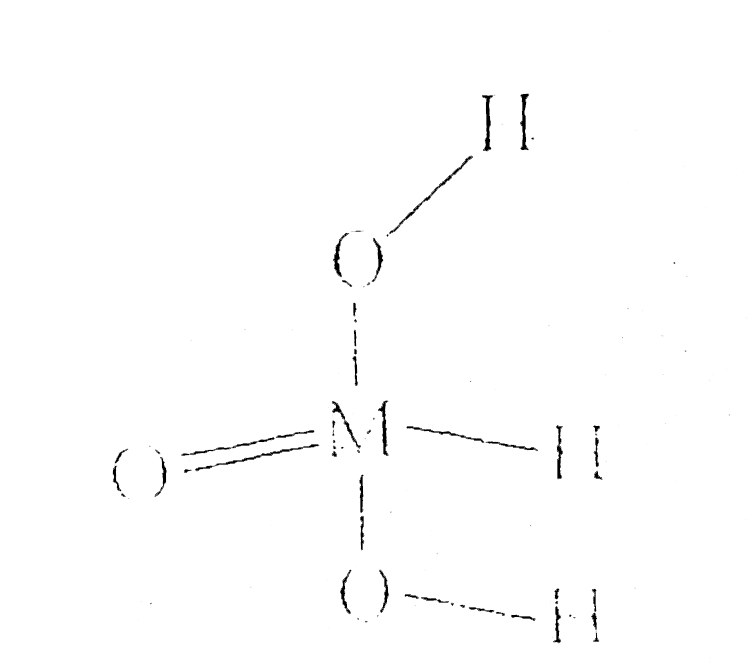
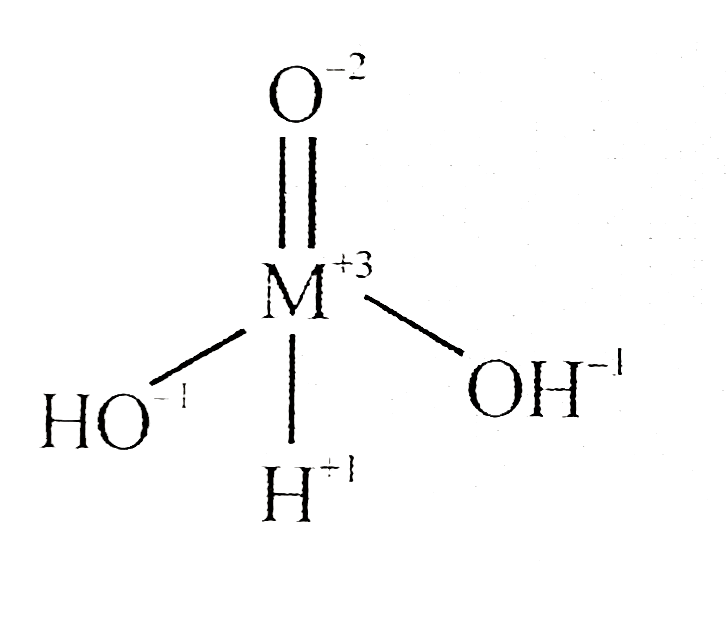A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- Among the following element which shows shows only 'one' non-zero oxid...
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|
- Identify the oxidation state of unknown element 'M' in following struc...
Text Solution
|
- Element which has minimum first ionisation energy is -
Text Solution
|
- Calculate the value of Z("eff") for 3d electron of .(21)Sc.
Text Solution
|
- The atomic numbers of vanasium (V) Chormium (Cr), manganese (Mn) and i...
Text Solution
|
- Select the incorrect order according to their given property -
Text Solution
|
- Identify the correct order of conductivity in an aqueous solution. ...
Text Solution
|
- Which of the following process is exothermic ?
Text Solution
|
- In which of the following arrangements the order is NOT according to t...
Text Solution
|
- Consider three hypothetical ionic compounds AB, A(2)B and A(2)B(3), w...
Text Solution
|
- For an element the successive ionisation energy value (in eV " atom"^(...
Text Solution
|
- The ionisation energies for B.Tl and In are X.Y and ZK cal/mol respect...
Text Solution
|
- Not considering the electronic spin, the degeneracy of the second exci...
Text Solution
|
- For an element having only valence shell electron, then which of the f...
Text Solution
|
- The set representing correct order of IP(1) is
Text Solution
|
- Arrange Ce^(3+),La^(3+), Pm^(3) and Yb^(3+) in increasing order of the...
Text Solution
|
- The increasing order of the first ionization enthalpies of the element...
Text Solution
|
- If the ionic radii of each K^(+) and F^(-) are 1.34Å, then tha atomic ...
Text Solution
|
- The difference in atomic numbers of the inert gas and alkali metal in ...
Text Solution
|