A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- The difference in atomic numbers of the inert gas and alkali metal in ...
Text Solution
|
- Select the correct order of electron affinity :
Text Solution
|
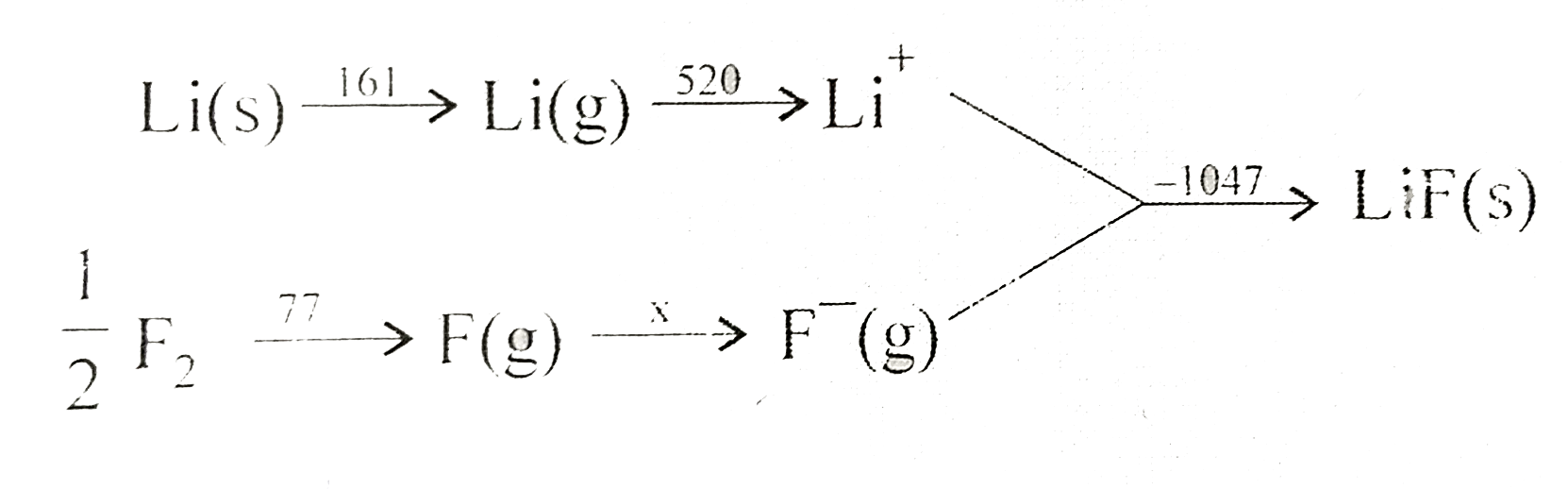
- Given {:("Reaction","Energy Change (in KJ)",),(Li(s) rarr Li(g),161,...
Text Solution
|
- How many number of elcetrons haven m = 0 value in chromium.
Text Solution
|
- Which of the following has highest bond dissociation energy ?
Text Solution
|
- Which is least acidic ?
Text Solution
|
- Which is amphoteric in nature ?
Text Solution
|
- Which of the following has mu = 0 dipole moment ?
Text Solution
|
- Which of the following has intramolecular H-bonding ?
Text Solution
|
- Which is correct order of electron affinity ?
Text Solution
|
- Which of the following orbital is not possible
Text Solution
|
- Choose incorrect order of ionic radii ?
Text Solution
|
- What is the basicity of phosphorus acid ?
Text Solution
|
- How many number of P - O - P linkage are present in tetrapolyphosphori...
Text Solution
|
- The incorrect set of the formal charge on different atoms in the Lewis...
Text Solution
|
- Select the incorrect statements from the following:
Text Solution
|
- Chose the correct bond angle order -
Text Solution
|
- Given the following information : A^(-)(g) rarr A^(2+) (g) + 3e^(-) ...
Text Solution
|
- In follwing compound which has minimum ionic radius of maganese is :
Text Solution
|
- Calculate total number of electron present in Cr having n + l + m = 6
Text Solution
|