A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
BANSAL-TEST PAPERS-CHEMISTRY PART-A
- The potential energy W of a system of two atoms A and B varies as a fu...
Text Solution
|
- The potential energy W of a system of two atoms A and B varies as a fu...
Text Solution
|
- The formal charge is the difference between the number of valence elec...
Text Solution
|
- The formal charge is the difference between the number of valence elec...
Text Solution
|
- How many number of tetraatomic species are planar ?
Text Solution
|
- Which of the following is /are sp^(3) hybridised with atleast one lone...
Text Solution
|
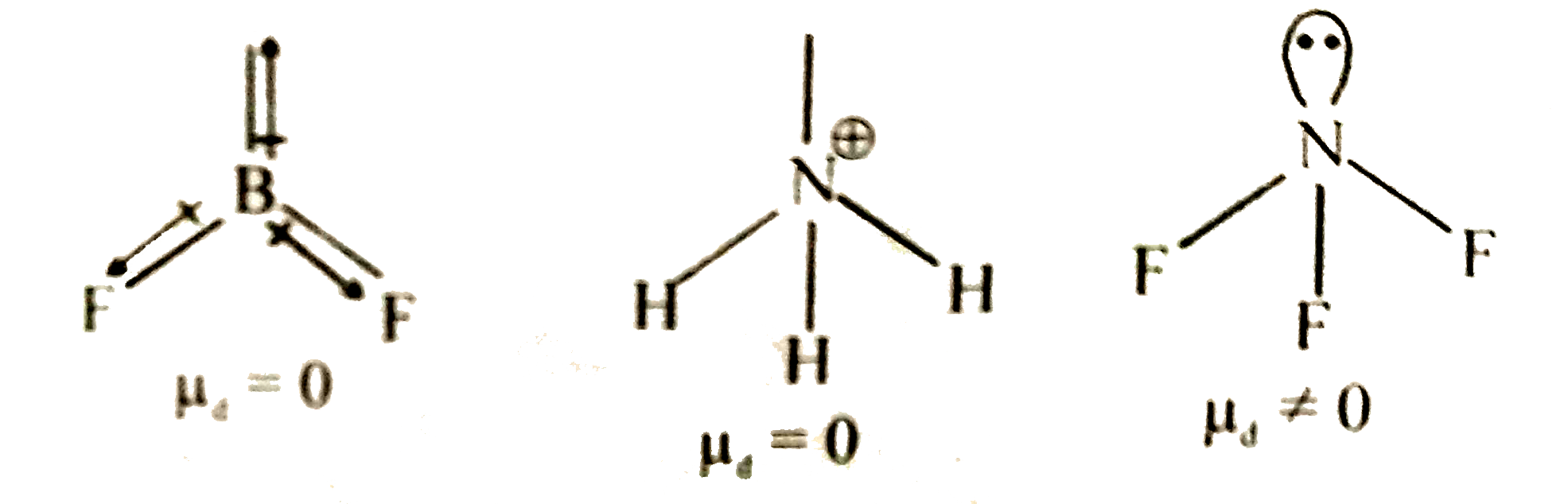
- Which of the following molecular species has/have mu = 0 dipole moment...
Text Solution
|
- Which options are correct for atomic radii ?
Text Solution
|
- Mendleeve left the space for elements in periodic table, the elements ...
Text Solution
|