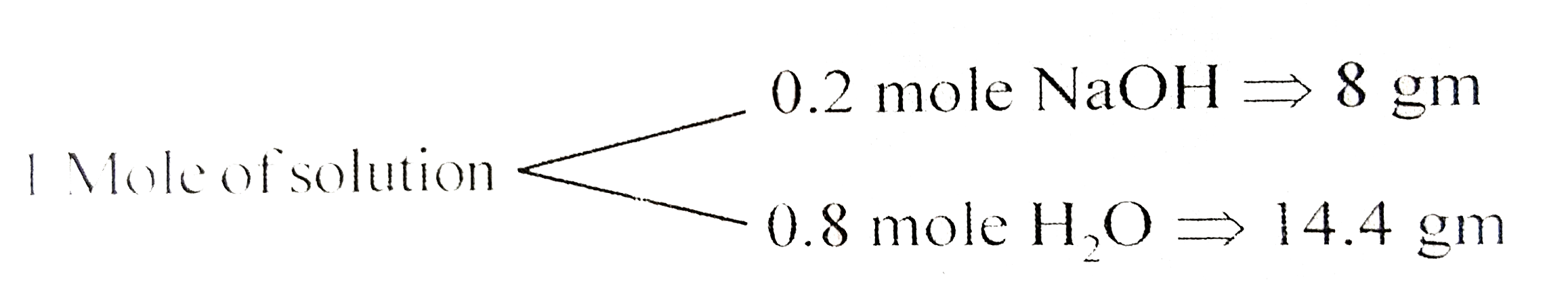A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- Which of the following has presence of three lone pair on centre atom ...
Text Solution
|
- 25.2 gm of a mixture of NaHCO(3) and Na(2)CO(3) is heated strongly, 0....
Text Solution
|
- The mole fraction of NaOH in its aqueous solution is 0.2 and density o...
Text Solution
|
- A gas mixture of CH(4) and C(3)H(6) undergo complete cracking into C(s...
Text Solution
|
- Which concentration term is temperature independent ?
Text Solution
|
- 10 moles of X, 12 mole of Y and 20 moles of Z are mixed to produce a f...
Text Solution
|
- 100 ml of 1 M H(2) SO(4) solution (d("solution") = 1.5 gm//ml) is mi...
Text Solution
|
- To find molelular mass of diabasic acid by silver salt method followin...
Text Solution
|
- 200 gm of an Oleum sample labeled as 109 % is mixed with 518 gm water ...
Text Solution
|
- An element A has three isotopes A^(20), A^(21), A^(22). The % abundanc...
Text Solution
|
- When 200 gm of an Oleum sample labeled as 109% is mixed with 300 gm of...
Text Solution
|
- An experiment is done as shown in diagram then final pressure after st...
Text Solution
|
- To form one molecule of Al(2)O(3) the toal number of electron transfer...
Text Solution
|
- For the reaction : 2 NH(3) (g) rarr N(2) (g) + 3H(2) (g). What is the ...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of Xenon ...
Text Solution
|
- A solution can be expressed as 25% w/w as well as 20% w/v then mass of...
Text Solution
|
- If Hund's rule is not followed then which of the following ion is para...
Text Solution
|
- Consider the following statements - (i) At same P, if V vs T graph i...
Text Solution
|
- Which of the following pair has same structure but different hybridisa...
Text Solution
|
- Consider the salt K(x) H(y) (C(2)O(4))(z).2H(2)O. The relationship bet...
Text Solution
|